Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-08-28 , DOI: 10.1016/j.bmcl.2020.127507 Julio César Medina-Rojas 1 , Irving Osiel Castillo-Rodríguez 1 , Elena Martínez-Klimova 2 , Teresa Ramírez-Ápan 1 , Simón Hernández-Ortega 1 , Marcos Martínez-García 1
|
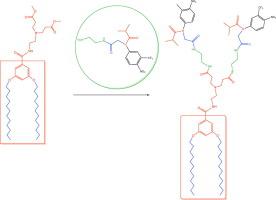
In this paper, we designed and extended modification basing on the flutamide structure. A series of flutamide-conjugates were obtained with methyl bromoacetate and ethylenediamine. Through the synthesis of two conjugates with 3,5-bis(dodecyloxy)benzoate derivatives, these flutamide conjugates were tested for anticancer activity. Among the compounds tested, the flutamide-conjugates showed good inhibition activity against cancer cell lines U-251, PC-3 and K-562. The conjugates showed a better inhibitory effect than free flutamide and did not show activity against normal COS-7 monkey kidney fibroblast cells. It was also observed that the flutamide conjugates had an inhibitory effect against human colorectal adenocarcinoma HCT-15.
中文翻译:

氟他胺-缀合物的合成。
在本文中,我们基于氟他胺的结构设计并扩展了修饰。用溴乙酸甲酯和乙二胺获得了一系列氟他胺结合物。通过合成具有3,5-双(十二烷基氧基)苯甲酸酯衍生物的两种缀合物,测试了这些氟他胺缀合物的抗癌活性。在所测试的化合物中,氟他胺结合物对癌细胞系U-251,PC-3和K-562表现出良好的抑制活性。结合物显示出比游离氟他胺更好的抑制作用,并且没有显示出对正常COS-7猴肾成纤维细胞的活性。还观察到氟他胺缀合物对人结肠直肠腺癌HCT-15具有抑制作用。

























 京公网安备 11010802027423号
京公网安备 11010802027423号