当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into How Protein Environments Tune the Spectroscopic Properties of a Noncanonical Amino Acid Fluorophore.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00474 J Nathan Henderson 1 , Chad R Simmons 1 , Nour Eddine Fahmi 1 , Joshua W Jeffs 2, 3 , Chad R Borges 2, 3 , Jeremy H Mills 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00474 J Nathan Henderson 1 , Chad R Simmons 1 , Nour Eddine Fahmi 1 , Joshua W Jeffs 2, 3 , Chad R Borges 2, 3 , Jeremy H Mills 1, 3
Affiliation
|
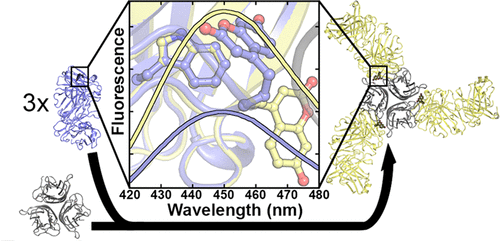
Genetically encoded fluorescent noncanonical amino acids (fNCAAs) could be used to develop novel fluorescent sensors of protein function. Previous efforts toward this goal have been limited by the lack of extensive physicochemical and structural characterizations of protein-based sensors containing fNCAAs. Here, we report the steady-state spectroscopic properties and first structural analyses of an fNCAA-containing Fab fragment of the 5c8 antibody, which binds human CD40L. A previously reported 5c8 variant in which the light chain residue IleL98 is replaced with the fNCAA l-(7-hydroxycoumarin-4-yl)ethylglycine (7-HCAA) exhibits a 1.7-fold increase in fluorescence upon antigen binding. Determination and comparison of the apparent pKas of 7-HCAA in the unbound and bound forms indicate that the observed increase in fluorescence is not the result of perturbations in pKa. Crystal structures of the fNCAA-containing Fab in the apo and bound forms reveal interactions between the 7-HCAA side chain and surrounding residues that are disrupted upon antigen binding. This structural characterization not only provides insight into the manner in which protein environments can modulate the fluorescence properties of 7-HCAA but also could serve as a starting point for the rational design of new fluorescent protein-based reporters of protein function.
中文翻译:

蛋白质环境如何调整非经典氨基酸荧光团的光谱特性的结构见解。
遗传编码的荧光非经典氨基酸 (fNCAA) 可用于开发蛋白质功能的新型荧光传感器。由于缺乏对含有 fNCAA 的基于蛋白质的传感器的广泛物理化学和结构表征,之前实现这一目标的努力受到了限制。在这里,我们报告了与人 CD40L 结合的 5c8 抗体的含 fNCAA Fab 片段的稳态光谱特性和首次结构分析。先前报告的5c8变体,其中异亮氨酸残基的轻链大号98被替换为fNCAA升- (7-羟基香豆素-4-基)乙基甘氨酸(7- HCAA)表现出荧光在抗原结合1.7倍的增加。表观 p K a 的测定和比较未结合和结合形式的 7-HCAA 的 s 表明观察到的荧光增加不是 p K a扰动的结果。apo 和结合形式中含有 fNCAA 的 Fab 的晶体结构揭示了 7-HCAA 侧链和周围残基之间的相互作用,这些残基在抗原结合时被破坏。这种结构表征不仅提供了对蛋白质环境调节 7-HCAA 荧光特性的方式的深入了解,而且还可以作为合理设计新的基于荧光蛋白的蛋白质功能报告基因的起点。
更新日期:2020-09-22
中文翻译:

蛋白质环境如何调整非经典氨基酸荧光团的光谱特性的结构见解。
遗传编码的荧光非经典氨基酸 (fNCAA) 可用于开发蛋白质功能的新型荧光传感器。由于缺乏对含有 fNCAA 的基于蛋白质的传感器的广泛物理化学和结构表征,之前实现这一目标的努力受到了限制。在这里,我们报告了与人 CD40L 结合的 5c8 抗体的含 fNCAA Fab 片段的稳态光谱特性和首次结构分析。先前报告的5c8变体,其中异亮氨酸残基的轻链大号98被替换为fNCAA升- (7-羟基香豆素-4-基)乙基甘氨酸(7- HCAA)表现出荧光在抗原结合1.7倍的增加。表观 p K a 的测定和比较未结合和结合形式的 7-HCAA 的 s 表明观察到的荧光增加不是 p K a扰动的结果。apo 和结合形式中含有 fNCAA 的 Fab 的晶体结构揭示了 7-HCAA 侧链和周围残基之间的相互作用,这些残基在抗原结合时被破坏。这种结构表征不仅提供了对蛋白质环境调节 7-HCAA 荧光特性的方式的深入了解,而且还可以作为合理设计新的基于荧光蛋白的蛋白质功能报告基因的起点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号