当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and Investigation of an Organocatalytic Enantioselective [10 + 2] Cycloaddition
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acscatal.0c03378 David McLeod 1 , Joseph A. Izzo 1 , Danny K. B. Jørgensen 1 , Rune F. Lauridsen 1 , Karl Anker Jørgensen 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acscatal.0c03378 David McLeod 1 , Joseph A. Izzo 1 , Danny K. B. Jørgensen 1 , Rune F. Lauridsen 1 , Karl Anker Jørgensen 1
Affiliation
|
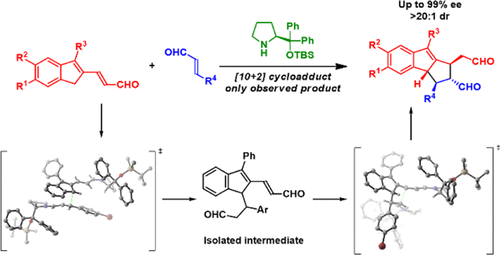
A stereoselective [10 + 2] cycloaddition for the reaction of homologated indenecarbaldehydes with α,β-unsaturated aldehydes, providing tetrahydrocyclopenta[a]indenes, has been developed and investigated mechanistically. The reaction proceeds via an aminocatalytic double Michael addition in high formal peri-, diastereo-, and enatioselectivity (up to 99% enantiomeric excess). Mechanistic investigations conclude that the reaction takes advantage of the in situ generation of a highly reactive amino isobenzofulvene intermediate via an aromative aminocatalytic strategy. A significant nonlinear effect is observed, consistent with a dual-activation model. Kinetic studies suggest a stepwise mechanism which is further supported by the identification and isolation of diastereomeric precyclization intermediates. These intermediates showed that in the presence of the aminocatalyst, they re-enter the catalytic cycle and afford the [10 + 2] cycloadduct with the same stereoselectivity observed in the prototypical reaction. Density functional theory calculations identified a Curtin–Hammett scenario where the stereoisomer of the [10 + 2] cycloadduct is determined by downstream species. These mechanistic investigations provide an understanding of the reaction pathway and stereoselectivity and continue to increase the knowledge of higher-order cycloadditions.
中文翻译:

有机催化对映选择性[10 + 2]环加成反应的研究与开发
已开发出立体选择的[10 + 2]环加成化合物,用于同价的茚满甲醛与α,β-不饱和醛反应,提供四氢环戊[ a ]茚,并进行了机理研究。该反应通过氨基催化双迈克尔加成反应以高形式的对映异构,非对映异构和对映选择性进行(最多99%对映体过量)。机理的研究得出结论,该反应利用了原位生成的高反应性的氨基isobenzofulvene中间经由芳香氨基催化策略。观察到明显的非线性效应,与双重激活模型一致。动力学研究提出了一种逐步的机制,其由非对映异构体预环化中间体的鉴定和分离进一步支持。这些中间体表明,在氨基催化剂的存在下,它们重新进入催化循环,并提供[10 + 2]环加合物,具有在原型反应中观察到的相同的立体选择性。密度泛函理论计算确定了Curtin-Hammett方案,其中[10 + 2]环加合物的立体异构体由下游物质决定。这些机理研究提供了对反应途径和立体选择性的理解,并继续增加了对高阶环加成反应的认识。
更新日期:2020-09-20
中文翻译:

有机催化对映选择性[10 + 2]环加成反应的研究与开发
已开发出立体选择的[10 + 2]环加成化合物,用于同价的茚满甲醛与α,β-不饱和醛反应,提供四氢环戊[ a ]茚,并进行了机理研究。该反应通过氨基催化双迈克尔加成反应以高形式的对映异构,非对映异构和对映选择性进行(最多99%对映体过量)。机理的研究得出结论,该反应利用了原位生成的高反应性的氨基isobenzofulvene中间经由芳香氨基催化策略。观察到明显的非线性效应,与双重激活模型一致。动力学研究提出了一种逐步的机制,其由非对映异构体预环化中间体的鉴定和分离进一步支持。这些中间体表明,在氨基催化剂的存在下,它们重新进入催化循环,并提供[10 + 2]环加合物,具有在原型反应中观察到的相同的立体选择性。密度泛函理论计算确定了Curtin-Hammett方案,其中[10 + 2]环加合物的立体异构体由下游物质决定。这些机理研究提供了对反应途径和立体选择性的理解,并继续增加了对高阶环加成反应的认识。


























 京公网安备 11010802027423号
京公网安备 11010802027423号