当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-glycome analysis detects dysglycosylation missed by conventional methods in SLC39A8 deficiency.
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-08-27 , DOI: 10.1002/jimd.12306 Julien H Park 1, 2 , Robert G Mealer 3, 4, 5 , Abdallah F Elias 6, 7 , Susanne Hoffmann 8 , Marianne Grüneberg 2 , Saskia Biskup 9 , Manfred Fobker 10 , Jaclyn Haven 6 , Ute Mangels 2 , Janine Reunert 2 , Stephan Rust 2 , Jonathan Schoof 6 , Corbin Schwanke 6 , Jordan W Smoller 3, 4 , Richard D Cummings 5 , Thorsten Marquardt 2
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-08-27 , DOI: 10.1002/jimd.12306 Julien H Park 1, 2 , Robert G Mealer 3, 4, 5 , Abdallah F Elias 6, 7 , Susanne Hoffmann 8 , Marianne Grüneberg 2 , Saskia Biskup 9 , Manfred Fobker 10 , Jaclyn Haven 6 , Ute Mangels 2 , Janine Reunert 2 , Stephan Rust 2 , Jonathan Schoof 6 , Corbin Schwanke 6 , Jordan W Smoller 3, 4 , Richard D Cummings 5 , Thorsten Marquardt 2
Affiliation
|
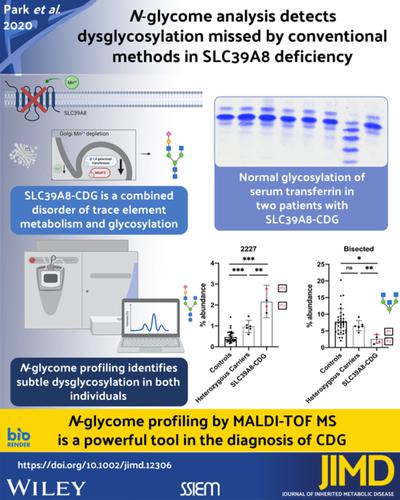
Congenital disorders of glycosylation (CDG) are a growing group of inborn metabolic disorders with multiorgan presentation. SLC39A8‐CDG is a severe subtype caused by biallelic mutations in the manganese transporter SLC39A8, reducing levels of this essential cofactor for many enzymes including glycosyltransferases. The current diagnostic standard for disorders of N‐glycosylation is the analysis of serum transferrin. Exome and Sanger sequencing were performed in two patients with severe neurodevelopmental phenotypes suggestive of CDG. Transferrin glycosylation was analyzed by high‐performance liquid chromatography (HPLC) and isoelectric focusing in addition to comprehensive N‐glycome analysis using matrix‐assisted laser desorption ionization time of flight (MALDI‐TOF) mass spectrometry (MS). Atomic absorption spectroscopy was used to quantify whole blood manganese levels. Both patients presented with a severe, multisystem disorder, and a complex neurological phenotype. Magnetic resonance imaging (MRI) revealed a Leigh‐like syndrome with bilateral T2 hyperintensities of the basal ganglia. In patient 1, exome sequencing identified the previously undescribed homozygous variant c.608T>C [p.F203S] in SLC39A8. Patient 2 was found to be homozygous for c.112G>C [p.G38R]. Both individuals showed a reduction of whole blood manganese, though transferrin glycosylation was normal. N‐glycome using MALDI‐TOF MS identified an increase of the asialo‐agalactosylated precursor N‐glycan A2G1S1 and a decrease in bisected structures. In addition, analysis of heterozygous CDG‐allele carriers identified similar but less severe glycosylation changes. Despite its reliance as a clinical gold standard, analysis of transferrin glycosylation cannot be categorically used to rule out SLC39A8‐CDG. These results emphasize that SLC39A8‐CDG presents as a spectrum of dysregulated glycosylation, and MS is an important tool for identifying deficiencies not detected by conventional methods.
中文翻译:

N-糖组分析检测 SLC39A8 缺陷中常规方法遗漏的糖基化异常。
先天性糖基化障碍 (CDG) 是一组越来越多的先天性代谢障碍,具有多器官表现。SLC39A8-CDG 是一种严重的亚型,由锰转运蛋白 SLC39A8 的双等位基因突变引起,降低了包括糖基转移酶在内的许多酶的这种必需辅因子的水平。目前N-糖基化障碍的诊断标准是血清转铁蛋白的分析。在两名具有提示 CDG 的严重神经发育表型的患者中进行了外显子组和 Sanger 测序。转铁蛋白糖基化通过高效液相色谱 (HPLC) 和等电聚焦以及综合N使用基质辅助激光解吸电离飞行时间 (MALDI-TOF) 质谱 (MS) 进行糖组分析。原子吸收光谱用于量化全血锰水平。两名患者都表现出严重的多系统疾病和复杂的神经表型。磁共振成像 (MRI) 显示 Leigh 样综合征伴双侧基底节 T2 高信号。在患者1,外显子测序确定了以前未描述的变体的纯合子c.608T> C [p.F203S] SLC39A8。发现患者 2 为 c.112G>C [p.G38R] 纯合子。尽管转铁蛋白糖基化正常,但两个人都显示出全血锰的减少。N-糖组使用 MALDI-TOF MS 确定了脱唾液酸-半乳糖基化前体N-聚糖 A2G1S1 的增加和二等分结构的减少。此外,对杂合 CDG 等位基因携带者的分析发现了相似但不太严重的糖基化变化。尽管它作为临床金标准的依赖,但转铁蛋白糖基化分析不能绝对用于排除 SLC39A8-CDG。这些结果强调,SLC39A8-CDG 表现为一系列糖基化失调,而 MS 是识别常规方法未检测到的缺陷的重要工具。
更新日期:2020-08-27
中文翻译:

N-糖组分析检测 SLC39A8 缺陷中常规方法遗漏的糖基化异常。
先天性糖基化障碍 (CDG) 是一组越来越多的先天性代谢障碍,具有多器官表现。SLC39A8-CDG 是一种严重的亚型,由锰转运蛋白 SLC39A8 的双等位基因突变引起,降低了包括糖基转移酶在内的许多酶的这种必需辅因子的水平。目前N-糖基化障碍的诊断标准是血清转铁蛋白的分析。在两名具有提示 CDG 的严重神经发育表型的患者中进行了外显子组和 Sanger 测序。转铁蛋白糖基化通过高效液相色谱 (HPLC) 和等电聚焦以及综合N使用基质辅助激光解吸电离飞行时间 (MALDI-TOF) 质谱 (MS) 进行糖组分析。原子吸收光谱用于量化全血锰水平。两名患者都表现出严重的多系统疾病和复杂的神经表型。磁共振成像 (MRI) 显示 Leigh 样综合征伴双侧基底节 T2 高信号。在患者1,外显子测序确定了以前未描述的变体的纯合子c.608T> C [p.F203S] SLC39A8。发现患者 2 为 c.112G>C [p.G38R] 纯合子。尽管转铁蛋白糖基化正常,但两个人都显示出全血锰的减少。N-糖组使用 MALDI-TOF MS 确定了脱唾液酸-半乳糖基化前体N-聚糖 A2G1S1 的增加和二等分结构的减少。此外,对杂合 CDG 等位基因携带者的分析发现了相似但不太严重的糖基化变化。尽管它作为临床金标准的依赖,但转铁蛋白糖基化分析不能绝对用于排除 SLC39A8-CDG。这些结果强调,SLC39A8-CDG 表现为一系列糖基化失调,而 MS 是识别常规方法未检测到的缺陷的重要工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号