当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical and experimental studies on concerted elimination of 1, 2‐bromochloroethane monocation to C2H4+ and BrCl
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-08-27 , DOI: 10.1002/qua.26433 Hua Wu 1 , Mengdi An 1 , Junqing Wen 1 , Lihua Bai 1 , Dongming Li 1 , Jukun Liu 2 , Ruijuan Sun 1 , Wanlin He 1 , Lin Lin 1 , Yumei Li 1
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-08-27 , DOI: 10.1002/qua.26433 Hua Wu 1 , Mengdi An 1 , Junqing Wen 1 , Lihua Bai 1 , Dongming Li 1 , Jukun Liu 2 , Ruijuan Sun 1 , Wanlin He 1 , Lin Lin 1 , Yumei Li 1
Affiliation
|
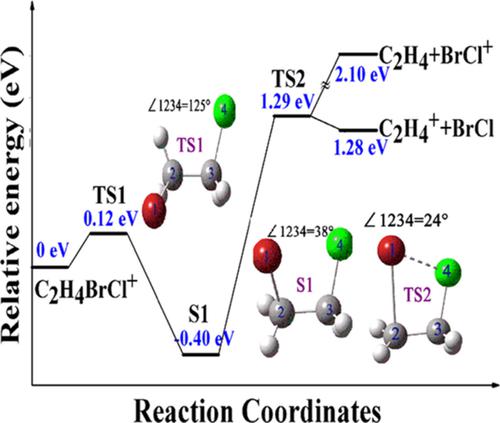
Ab initio calculations for the concerted elimination of 1, 2‐bromochloroethane monocation (1,2‐C2H4BrCl+) to C2H4+ and BrCl are performed using the Minnesota density functional M06‐2X and the def2‐TZVP basis set. Ab initio calculations are also carried out for the concerted elimination of 1,2‐C2H4BrCl+ to C2H4 and BrCl+ because the positive charge can be assigned to either moiety in the dissociative ionization process of 1,2‐C2H4BrCl+. Our results demonstrate that the concerted elimination channel of 1,2‐C2H4BrCl+ to C2H4+ and BrCl is preferred and that 1,2‐C2H4BrCl+ surpasses two energy barriers and then separates into C2H4+ + BrCl through an asynchronous concerted process. Experimentally, we confirm that this elimination channel is from the dissociative ionization process of 1,2‐C2H4BrCl+ by using dc‐slice imaging technique. The time‐of‐flight mass spectra of 1,2‐bromochloroethane induced by femtosecond laser pulses show that C2H4+ occurs at a laser intensity of 6.0 × 1013 W/cm2, and BrCl+ occurs at a higher laser intensity than C2H4+. This finding is consistent with the theoretical result that the appearance energy of C2H4+ is lower than that of BrCl+. As such, the low‐velocity component of BrCl+ is absent from our sliced images.
中文翻译:

协同消除1,2-溴氯乙烷单阳离子成C2H4 +和BrCl的理论和实验研究
使用明尼苏达州密度函数M06-2X和def2-TZVP基础进行从头算计算,以协同消除1,2-溴氯乙烷单阳离子(1,2-C 2 H 4 BrCl +)为C 2 H 4 +和BrCl组。还进行了从头算计算以协调消除1,2-C 2 H 4 BrCl +到C 2 H 4和BrCl +,因为在1,2-的解离电离过程中可以将正电荷分配给任一部分C 2 H 4 BrCl +。我们的结果表明,首选的1,2-C 2 H 4 BrCl +协同消除通道为C 2 H 4 +和BrCl,并且1,2-C 2 H 4 BrCl +超过两个能垒,然后分离为C 2 H 4 + + BrCl通过异步协同过程进行。通过实验,我们确认该消除通道来自1,2-C 2 H 4 BrCl +的解离电离过程通过使用直流切片成像技术。飞秒激光脉冲诱导的1,2-溴氯乙烷的飞行时间质谱表明,C 2 H 4 +发生在6.0×10 13 W / cm 2的激光强度下,而BrCl +发生在较高的激光强度下比C 2 H 4 +。这一发现与C 2 H 4 +的表观能量低于BrCl +的表观能量的理论结果一致。因此,切片图像中不存在BrCl +的低速度成分。
更新日期:2020-08-27
中文翻译:

协同消除1,2-溴氯乙烷单阳离子成C2H4 +和BrCl的理论和实验研究
使用明尼苏达州密度函数M06-2X和def2-TZVP基础进行从头算计算,以协同消除1,2-溴氯乙烷单阳离子(1,2-C 2 H 4 BrCl +)为C 2 H 4 +和BrCl组。还进行了从头算计算以协调消除1,2-C 2 H 4 BrCl +到C 2 H 4和BrCl +,因为在1,2-的解离电离过程中可以将正电荷分配给任一部分C 2 H 4 BrCl +。我们的结果表明,首选的1,2-C 2 H 4 BrCl +协同消除通道为C 2 H 4 +和BrCl,并且1,2-C 2 H 4 BrCl +超过两个能垒,然后分离为C 2 H 4 + + BrCl通过异步协同过程进行。通过实验,我们确认该消除通道来自1,2-C 2 H 4 BrCl +的解离电离过程通过使用直流切片成像技术。飞秒激光脉冲诱导的1,2-溴氯乙烷的飞行时间质谱表明,C 2 H 4 +发生在6.0×10 13 W / cm 2的激光强度下,而BrCl +发生在较高的激光强度下比C 2 H 4 +。这一发现与C 2 H 4 +的表观能量低于BrCl +的表观能量的理论结果一致。因此,切片图像中不存在BrCl +的低速度成分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号