当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The catalytic performance of metal‐free defected carbon catalyst towards acetylene hydrochlorination revealed from first‐principles calculation
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-08-26 , DOI: 10.1002/qua.26418 Sajjad Ali 1, 2 , Muhammad Baber Azam Khan 3 , Said Alam Khan 4 , Noora 5
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-08-26 , DOI: 10.1002/qua.26418 Sajjad Ali 1, 2 , Muhammad Baber Azam Khan 3 , Said Alam Khan 4 , Noora 5
Affiliation
|
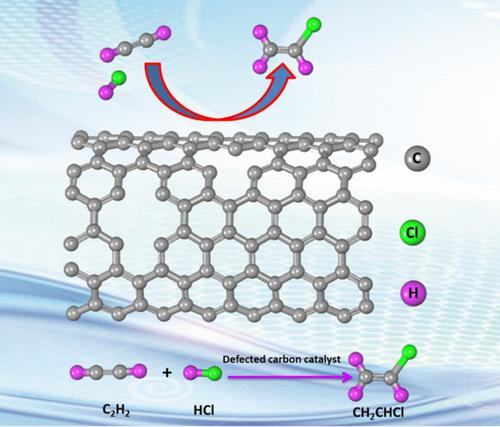
Defected carbon materials as a metal‐free catalyst have shown superior stability and catalytic performance in the acetylene hydrochlorination reaction. Through density functional theory (DFT) calculations, for the first time, several different defected configurations comprising mono and divacancies and Stone Wales defect on single‐walled carbon nanotubes (SWCNTs) have been used as a direct catalyst for acetylene hydrochlorination reaction. These defective sites on SWCNTs are the most active site for acetylene hydrochlorination reaction compare to pristine SWCNT. The different configurations of defects have different electronic structures, which specify that monovacancy defects have more states adjacent to the Fermi level. The reactant acetylene (C2H2) adsorbed strongly compared to hydrogen chloride (HCl) and expected to be the initial step of the reaction. Acetylene adsorbed strongly at monovacancy defected SWCNT compared to other investigated defects. Reaction pathway analysis revealed that mono‐ and divacancy defected SWCNTs have minimum energy barriers and show extraordinary performance toward acetylene hydrochlorination. This work suggests the potential of metal‐free defected carbon in catalyzing acetylene hydrochlorination and provides a solid base for future developments in acetylene hydrochlorination.
中文翻译:

从第一性原理计算可知,无金属缺陷碳催化剂对乙炔盐酸的催化性能
缺陷碳材料作为无金属催化剂在乙炔氢氯化反应中显示出优异的稳定性和催化性能。通过密度泛函理论(DFT)的计算,单壁碳纳米管(SWCNTs)上的几种不同的缺陷结构(包括单空位和双空位以及Stone Wales缺陷)首次被用作乙炔氢氯化反应的直接催化剂。与原始SWCNT相比,SWCNT上的这些缺陷位点是乙炔氢氯化反应最活跃的位点。缺陷的不同配置具有不同的电子结构,这指定单空位缺陷在费米能级附近具有更多的状态。反应物乙炔(C 2 H 2)与氯化氢(HCl)相比,吸附力强,有望成为反应的第一步。与其他研究的缺陷相比,乙炔在单空位缺陷的SWCNT处被强烈吸附。反应路径分析表明,单空位和双空位缺陷的SWCNT具有最小的能量壁垒,并且在乙炔氢氯化反应中显示出非凡的性能。这项工作表明了无金属缺陷碳在催化乙炔氢氯化反应中的潜力,并为乙炔氢氯化反应的未来发展提供了坚实的基础。
更新日期:2020-08-26
中文翻译:

从第一性原理计算可知,无金属缺陷碳催化剂对乙炔盐酸的催化性能
缺陷碳材料作为无金属催化剂在乙炔氢氯化反应中显示出优异的稳定性和催化性能。通过密度泛函理论(DFT)的计算,单壁碳纳米管(SWCNTs)上的几种不同的缺陷结构(包括单空位和双空位以及Stone Wales缺陷)首次被用作乙炔氢氯化反应的直接催化剂。与原始SWCNT相比,SWCNT上的这些缺陷位点是乙炔氢氯化反应最活跃的位点。缺陷的不同配置具有不同的电子结构,这指定单空位缺陷在费米能级附近具有更多的状态。反应物乙炔(C 2 H 2)与氯化氢(HCl)相比,吸附力强,有望成为反应的第一步。与其他研究的缺陷相比,乙炔在单空位缺陷的SWCNT处被强烈吸附。反应路径分析表明,单空位和双空位缺陷的SWCNT具有最小的能量壁垒,并且在乙炔氢氯化反应中显示出非凡的性能。这项工作表明了无金属缺陷碳在催化乙炔氢氯化反应中的潜力,并为乙炔氢氯化反应的未来发展提供了坚实的基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号