当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rigid Scaffolds: Synthesis of 2,6-Bridged Piperazines with Functional Groups in all three Bridges.
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-08-27 , DOI: 10.1002/open.202000188 Donglin Gao 1 , Christian Penno 1 , Bernhard Wünsch 1, 2
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-08-27 , DOI: 10.1002/open.202000188 Donglin Gao 1 , Christian Penno 1 , Bernhard Wünsch 1, 2
Affiliation
|
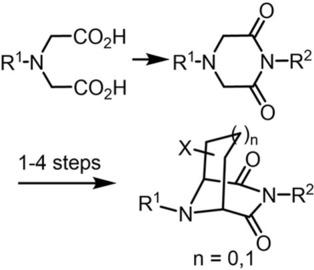
The activity of pharmacologically active compounds can be increased by presenting a drug in a defined conformation, which fits exactly into the binding pocket of its target. Herein, the piperazine scaffold was conformationally restricted by substituted C2‐ or C3‐bridges across the 2‐ and 6‐position. At first, a three‐step, one‐pot procedure was developed to obtain reproducibly piperazine‐2,6‐diones with various substituents at the N‐atoms in high yields. Three strategies for bridging of piperazine‐2,6‐diones were pursued: 1. The bicyclic mixed ketals 8‐benzyl‐6‐ethoxy‐3‐(4‐methoxybenzyl)‐6‐(trimethylsilyloxy)‐3,8‐diazabicyclo[3.2.1]octane‐2,4‐diones were prepared by Dieckmann analogous cyclization of 2‐(3,5‐dioxopiperazin‐2‐yl)acetates. 2. Stepwise allylation, hydroboration and oxidation of piperazine‐2,6‐diones led to 3‐(3,5‐dioxopiperazin‐2‐yl)propionaldehydes. Whereas reaction of such an aldehyde with base provided the bicyclic alcohol 9‐benzyl‐6‐hydroxy‐3‐(4‐methoxybenzyl)‐3,9‐diazabicyclo[3.3.1]nonane‐2,4‐dione in only 10 % yield, the corresponding sulfinylimines reacted with base to give N‐(2,4‐dioxo‐3,9‐diazabicyclo[3.3.1]nonan‐6‐yl)‐2‐methylpropane‐2‐sulfinamides in >66 % yield. 3. Transformation of a piperazine‐2,6‐dione with 1,4‐dibromobut‐2‐ene and 3‐halo‐2‐halomethylprop‐1‐enes provided 3,8‐diazabicyclo[3.2.1]octane‐2,4‐dione and 3,9‐diazabicyclo[3.3.1]nonane‐2,4‐dione with a vinyl group at the C2‐ or a methylene group at the C3‐bridge, respectively. Since bridging via sulfinylimines and the one‐pot bridging with 3‐bromo‐2‐bromomethylprop‐1‐ene gave promising yields, these strategies will be exploited for the synthesis of novel receptor ligands bearing various substituents in a defined orientation at the carbon bridge
中文翻译:

刚性支架:在所有三个桥中均具有官能团的 2,6-桥连哌嗪的合成。
药理活性化合物的活性可以通过以确定的构象呈现药物来增加,该构象恰好适合其靶标的结合口袋。在此,哌嗪支架受取代的 C 2 - 或 C 3构象限制-跨越2-和6-位置的桥梁。首先,开发了三步一锅法以高产率获得在 N 原子上具有各种取代基的可重复哌嗪-2,6-二酮。对哌嗪-2,6-二酮桥接的三种策略进行了探索:1.双环混合缩酮8-苄基-6-乙氧基-3-(4-甲氧基苄基)-6-(三甲基甲硅烷氧基)-3,8-二氮杂双环[3.2 .1]辛烷-2,4-二酮是通过 2-(3,5-二氧哌嗪-2-基)乙酸酯的 Dieckmann 类似环化制备的。2. 哌嗪-2,6-二酮的逐步烯丙基化、硼氢化和氧化生成3-(3,5-二氧哌嗪-2-基)丙醛。而这种醛与碱的反应提供了双环醇 9-苄基-6-羟基-3-(4-甲氧基苄基)-3,9-二氮杂双环[3.3.1]壬烷-2,4-二酮,产率仅为 10% ,相应的亚磺酰亚胺与碱反应得到N- (2,4-dioxo-3,9-diazabicyclo[3.3.1]nonan-6-yl)-2-methylpropane-2-sulfinamides,产率>66%。3.哌嗪-2,6-二酮与1,4-二溴丁-2-烯和3-卤-2-卤甲基丙-1-烯的转化得到3,8-二氮杂双环[3.2.1]辛烷-2,4 -二酮和 3,9-二氮杂双环[3.3.1]壬烷-2,4-二酮,分别在 C 2 - 或 C 3 -桥上有一个亚甲基。由于通过亚磺酰亚胺进行桥接和与 3-溴-2-溴甲基丙-1-烯的单锅桥接具有良好的产率,因此这些策略将用于合成在碳桥上具有特定方向的各种取代基的新型受体配体
更新日期:2020-08-27
中文翻译:

刚性支架:在所有三个桥中均具有官能团的 2,6-桥连哌嗪的合成。
药理活性化合物的活性可以通过以确定的构象呈现药物来增加,该构象恰好适合其靶标的结合口袋。在此,哌嗪支架受取代的 C 2 - 或 C 3构象限制-跨越2-和6-位置的桥梁。首先,开发了三步一锅法以高产率获得在 N 原子上具有各种取代基的可重复哌嗪-2,6-二酮。对哌嗪-2,6-二酮桥接的三种策略进行了探索:1.双环混合缩酮8-苄基-6-乙氧基-3-(4-甲氧基苄基)-6-(三甲基甲硅烷氧基)-3,8-二氮杂双环[3.2 .1]辛烷-2,4-二酮是通过 2-(3,5-二氧哌嗪-2-基)乙酸酯的 Dieckmann 类似环化制备的。2. 哌嗪-2,6-二酮的逐步烯丙基化、硼氢化和氧化生成3-(3,5-二氧哌嗪-2-基)丙醛。而这种醛与碱的反应提供了双环醇 9-苄基-6-羟基-3-(4-甲氧基苄基)-3,9-二氮杂双环[3.3.1]壬烷-2,4-二酮,产率仅为 10% ,相应的亚磺酰亚胺与碱反应得到N- (2,4-dioxo-3,9-diazabicyclo[3.3.1]nonan-6-yl)-2-methylpropane-2-sulfinamides,产率>66%。3.哌嗪-2,6-二酮与1,4-二溴丁-2-烯和3-卤-2-卤甲基丙-1-烯的转化得到3,8-二氮杂双环[3.2.1]辛烷-2,4 -二酮和 3,9-二氮杂双环[3.3.1]壬烷-2,4-二酮,分别在 C 2 - 或 C 3 -桥上有一个亚甲基。由于通过亚磺酰亚胺进行桥接和与 3-溴-2-溴甲基丙-1-烯的单锅桥接具有良好的产率,因此这些策略将用于合成在碳桥上具有特定方向的各种取代基的新型受体配体



























 京公网安备 11010802027423号
京公网安备 11010802027423号