当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of benorilate in twelve monosolvents: Determination, correlation and COSMO-RS analysis
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106272 Weiguo Hu , Zeren Shang , Ning Wei , Baohong Hou , Junbo Gong , Yan Wang
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106272 Weiguo Hu , Zeren Shang , Ning Wei , Baohong Hou , Junbo Gong , Yan Wang
|
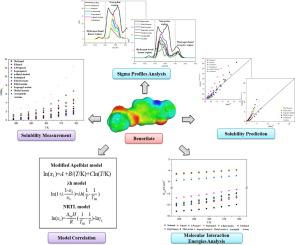
Abstract The solubility of benorilate in twelve monosolvents (methanol, ethanol, n-propanol, isopropanol, n-butyl alcohol, isobutanol, ethyl formate, ethyl acetate, isopropyl acetate, methyl acetate, acetonitrile and acetone) was determined at temperatures ranging from 278.15 K to 318.15 K using a static method under atmospheric pressure. The solubility trend of benorilate has two features, one is that the solubility increases with rising temperature, the other is that benorilate generally has larger solubility in dipolar aprotic solvents than in polar protic solvents. Furthermore, three classical thermodynamic models (modified Apelblat model, λh model and NRTL model) were used to correlate these determined solubility data, and modified Apelblat model shows the minor deviation than other models. Moreover, three solution thermodynamic parameters of mixing process were analyzed on the basis of NRTL model, and the mixing process was found to be spontaneous and entropy-driven. This work also used COSMO-RS model to predict reasonable solubility data of benorilate with experimental thermal analysis method and reference solubility method respectively. The underlying mechanism of benorilate and solvent molecules in solubility behavior was qualitatively analyzed by physicochemical properties and sigma profiles of the studied systems. The molecular interaction energies of benorilate in the studied solvents were computed on the basis of COSMO-RS model, and the computed values were also used to quantitatively analyze interactions in solutions of benorilate. The results obtained by qualitative and quantitative analysis demonstrate that the solvation of benorilate is a complicated process which is subject to the combined effects of a variety of molecular interactions. This research could provide guidance for the crystallization process optimization of benorilate.
中文翻译:

苯甲酸酯在十二种单溶剂中的溶解度:测定、相关性和 COSMO-RS 分析
摘要 苯甲酸酯在十二种单溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、甲酸乙酯、乙酸乙酯、乙酸异丙酯、乙酸甲酯、乙腈和丙酮)中的溶解度在 278.15 K 的温度范围内测定在大气压下使用静态方法升至 318.15 K。Benorilate的溶解度趋势有两个特点,一是溶解度随温度升高而增加,二是benorilate在偶极非质子溶剂中的溶解度一般大于在极性质子溶剂中的溶解度。此外,三个经典热力学模型(改进的 Apelblat 模型、λh 模型和 NRTL 模型)用于关联这些确定的溶解度数据,并且改进的 Apelblat 模型显示出与其他模型相比的微小偏差。而且,基于NRTL模型分析了混合过程的三个溶液热力学参数,发现混合过程是自发的和熵驱动的。本工作还利用COSMO-RS模型分别用实验热分析法和参考溶解度法预测了苯甲酸的合理溶解度数据。通过所研究系统的理化性质和西格玛曲线,定性分析了苯甲酸酯和溶剂分子在溶解性行为中的潜在机制。基于COSMO-RS模型计算了苯甲酸酯在所研究溶剂中的分子相互作用能,并利用计算值定量分析了苯甲酸酯溶液中的相互作用。定性和定量分析结果表明苯甲酸酯的溶剂化是一个复杂的过程,受多种分子相互作用的综合影响。该研究可为苯甲酸酯的结晶工艺优化提供指导。
更新日期:2021-01-01
中文翻译:

苯甲酸酯在十二种单溶剂中的溶解度:测定、相关性和 COSMO-RS 分析
摘要 苯甲酸酯在十二种单溶剂(甲醇、乙醇、正丙醇、异丙醇、正丁醇、异丁醇、甲酸乙酯、乙酸乙酯、乙酸异丙酯、乙酸甲酯、乙腈和丙酮)中的溶解度在 278.15 K 的温度范围内测定在大气压下使用静态方法升至 318.15 K。Benorilate的溶解度趋势有两个特点,一是溶解度随温度升高而增加,二是benorilate在偶极非质子溶剂中的溶解度一般大于在极性质子溶剂中的溶解度。此外,三个经典热力学模型(改进的 Apelblat 模型、λh 模型和 NRTL 模型)用于关联这些确定的溶解度数据,并且改进的 Apelblat 模型显示出与其他模型相比的微小偏差。而且,基于NRTL模型分析了混合过程的三个溶液热力学参数,发现混合过程是自发的和熵驱动的。本工作还利用COSMO-RS模型分别用实验热分析法和参考溶解度法预测了苯甲酸的合理溶解度数据。通过所研究系统的理化性质和西格玛曲线,定性分析了苯甲酸酯和溶剂分子在溶解性行为中的潜在机制。基于COSMO-RS模型计算了苯甲酸酯在所研究溶剂中的分子相互作用能,并利用计算值定量分析了苯甲酸酯溶液中的相互作用。定性和定量分析结果表明苯甲酸酯的溶剂化是一个复杂的过程,受多种分子相互作用的综合影响。该研究可为苯甲酸酯的结晶工艺优化提供指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号