Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-27 , DOI: 10.1016/j.tet.2020.131519 Qin Yang , Yang Zhu , Guisheng Deng
|
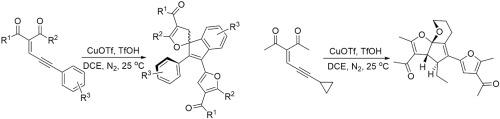
CuOTf/TfOH-mediated cascade cyclization-coupling-electrophilic substitution of conjugated ene-yne-ketones in DCE at 25 °C provided novel spiro dihydrofurans in 32–83% yield. The experimental results demonstrated that substituent group R3, which is electron-donated or electron-withdrawing group, decreased the yield. Additionally, significant effects of R2 group adjacent to carbonyl carbon on reactivity and yield of the reaction were also observed. Both the reactivity and yield were decreased when replacing methyl group or ethyl group with bulky substituent group (e.g., aryl group, cyclopropyl group). For 3-(3-cyclopropylprop-2-yn-1-ylidene)pentane-2,4-dione, tetrahydro-6H-furo[2′,3’:1,5]cyclopenta[1,2-b]pyran was generated. This method proved to be simple and mild.
中文翻译:

CuOTf / TfOH介导的共轭烯-炔酮的串联反应:新型螺二氢呋喃的合成
CuOTf / TfOH介导的DCE中25°C的共轭烯-炔-酮的级联环化-偶联-亲电取代提供了新型螺二氢呋喃,收率为32-83%。实验结果表明,给电子或吸电子基团的取代基R 3降低了产率。另外,还观察到与羰基碳相邻的R 2基团对反应性和反应产率的显着影响。当用大的取代基(例如芳基,环丙基)取代甲基或乙基时,反应性和产率均降低。对于3-(3-环丙基丙-2-炔-1-基)戊烷-2,4-二酮,四氢-6H-呋喃[2',3':1,5]环戊[1,2-b]吡喃为产生。这种方法被证明是简单而温和的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号