当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemically enabled synthesis of sulfide imidazopyridines via a radical cyclization cascade
Green Chemistry ( IF 9.8 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0gc02125c Ping-Fu Zhong 1, 2, 3, 4 , Hong-Min Lin 1, 2, 3, 4 , Lin-Wei Wang 1, 2, 3, 4 , Zu-Yu Mo 1, 2, 3, 4 , Xiu-Jin Meng 1, 2, 3, 4 , Hai-Tao Tang 1, 2, 3, 4 , Ying-Ming Pan 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0gc02125c Ping-Fu Zhong 1, 2, 3, 4 , Hong-Min Lin 1, 2, 3, 4 , Lin-Wei Wang 1, 2, 3, 4 , Zu-Yu Mo 1, 2, 3, 4 , Xiu-Jin Meng 1, 2, 3, 4 , Hai-Tao Tang 1, 2, 3, 4 , Ying-Ming Pan 1, 2, 3, 4
Affiliation
|
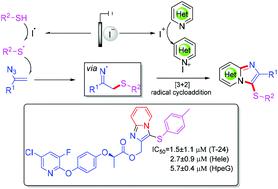
Owing to the inert nature of the pyridine ring and high activity of iminyl radicals, the reaction between pyridine and iminyl radicals remains a significant challenge. In this paper, we report the synthesis of sulfide imidazo[1,2-a]pyridines from vinyl azides, thiophenols, and pyridines via a radical [3 + 2] cycloaddition. Promoting inert pyridine and highly active iminyl radicals to participate in this intermolecular cycloaddition process is the striking feature of this protocol. The excellent antitumor activity of the prepared sulfide imidazopyridine scaffold demonstrated the synthetic utility of the developed synthetic protocol.
中文翻译:

通过自由基环化级联的电化学合成硫化物咪唑并吡啶
由于吡啶环的惰性和亚氨基自由基的高活性,吡啶和亚氨基自由基之间的反应仍然是一个重大挑战。在本文中,我们报告了通过自由基[3 + 2]环加成反应从乙烯基叠氮化物,硫酚和吡啶合成硫化物咪唑并[1,2- a ]吡啶。促进惰性吡啶和高活性亚氨基自由基参与该分子间环加成过程是该方案的显着特征。所制备的硫化物咪唑并吡啶支架的优异抗肿瘤活性证明了开发的合成方案的合成实用性。
更新日期:2020-10-05
中文翻译:

通过自由基环化级联的电化学合成硫化物咪唑并吡啶
由于吡啶环的惰性和亚氨基自由基的高活性,吡啶和亚氨基自由基之间的反应仍然是一个重大挑战。在本文中,我们报告了通过自由基[3 + 2]环加成反应从乙烯基叠氮化物,硫酚和吡啶合成硫化物咪唑并[1,2- a ]吡啶。促进惰性吡啶和高活性亚氨基自由基参与该分子间环加成过程是该方案的显着特征。所制备的硫化物咪唑并吡啶支架的优异抗肿瘤活性证明了开发的合成方案的合成实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号