当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06502 Alja Prah 1, 2 , Miha Purg 3 , Jernej Stare 1 , Robert Vianello 4 , Janez Mavri 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06502 Alja Prah 1, 2 , Miha Purg 3 , Jernej Stare 1 , Robert Vianello 4 , Janez Mavri 1
Affiliation
|
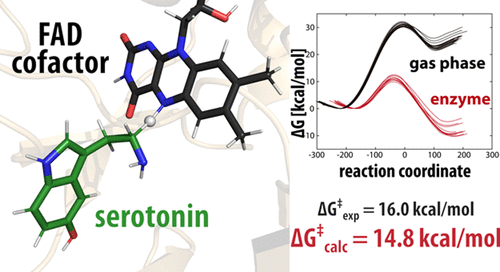
The enzyme-catalyzed degradation of the biogenic amine serotonin is an essential regulatory mechanism of its level in the human organism. In particular, monoamine oxidase A (MAO A) is an important flavoenzyme involved in the metabolism of monoamine neurotransmitters. Despite extensive research efforts, neither the catalytic nor the inhibition mechanisms of MAO enzymes are currently fully understood. In this article, we present the quantum mechanics/molecular mechanics simulation of the rate-limiting step for the serotonin decomposition, which consists of hydride transfer from the serotonin methylene group to the N5 atom of the flavin moiety. Free-energy profiles of the reaction were computed by the empirical valence bond method. Apart from the enzymatic environment, the reference reaction in the gas phase was also simulated, facilitating the estimation of the catalytic effect of the enzyme. The calculated barrier for the enzyme-catalyzed reaction of 14.82 ± 0.81 kcal mol–1 is in good agreement with the experimental value of 16.0 kcal mol–1, which provides strong evidence for the validity of the proposed hydride-transfer mechanism. Together with additional experimental and computational work, the results presented herein contribute to a deeper understanding of the catalytic mechanism of MAO A and flavoenzymes in general, and in the long run, they should pave the way toward applications in neuropsychiatry.
中文翻译:

单胺氧化酶A如何分解5-羟色胺:反应步骤的经验价键模拟。
生物胺5-羟色胺的酶催化降解是其在人体中水平的重要调节机制。特别地,单胺氧化酶A(MAO A)是参与单胺神经递质代谢的重要黄素酶。尽管进行了广泛的研究,但目前尚未完全理解MAO酶的催化或抑制机理。在本文中,我们介绍了5-羟色胺分解限速步骤的量子力学/分子力学模拟,该过程包括从5-羟色胺亚甲基到黄素部分的N5原子的氢化物转移。通过经验价键法计算反应的自由能分布。除了酶促环境外,还模拟了气相中的参考反应,有助于估计酶的催化作用。计算得出的酶催化反应的势垒为14.82±0.81 kcal mol–1与16.0 kcal mol –1的实验值非常吻合,这为所提出的氢化物转移机理的有效性提供了有力的证据。连同其他实验和计算工作,本文给出的结果有助于更深入地理解MAO A和黄酮酶的催化机理,从长远来看,它们应为神经精神病学的应用铺平道路。
更新日期:2020-09-24
中文翻译:

单胺氧化酶A如何分解5-羟色胺:反应步骤的经验价键模拟。
生物胺5-羟色胺的酶催化降解是其在人体中水平的重要调节机制。特别地,单胺氧化酶A(MAO A)是参与单胺神经递质代谢的重要黄素酶。尽管进行了广泛的研究,但目前尚未完全理解MAO酶的催化或抑制机理。在本文中,我们介绍了5-羟色胺分解限速步骤的量子力学/分子力学模拟,该过程包括从5-羟色胺亚甲基到黄素部分的N5原子的氢化物转移。通过经验价键法计算反应的自由能分布。除了酶促环境外,还模拟了气相中的参考反应,有助于估计酶的催化作用。计算得出的酶催化反应的势垒为14.82±0.81 kcal mol–1与16.0 kcal mol –1的实验值非常吻合,这为所提出的氢化物转移机理的有效性提供了有力的证据。连同其他实验和计算工作,本文给出的结果有助于更深入地理解MAO A和黄酮酶的催化机理,从长远来看,它们应为神经精神病学的应用铺平道路。

























 京公网安备 11010802027423号
京公网安备 11010802027423号