当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atroposelective Access to Oxindole-Based Axially Chiral Styrenes via the Strategy of Catalytic Kinetic Resolution
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-26 , DOI: 10.1021/jacs.0c00208 Chun Ma 1 , Feng-Tao Sheng 1 , Hai-Qing Wang 1 , Shuang Deng 2 , Yu-Chen Zhang 1 , Yinchun Jiao 2 , Wei Tan 1 , Feng Shi 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-26 , DOI: 10.1021/jacs.0c00208 Chun Ma 1 , Feng-Tao Sheng 1 , Hai-Qing Wang 1 , Shuang Deng 2 , Yu-Chen Zhang 1 , Yinchun Jiao 2 , Wei Tan 1 , Feng Shi 1
Affiliation
|
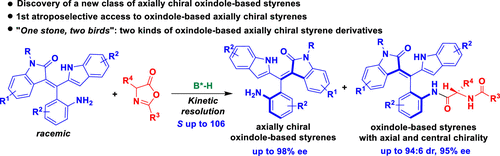
Atroposelective synthesis of axially chiral molecules has attracted substantial attention from chemists because of the importance of such molecules. However, catalytic asymmetric synthesis of axially chiral styrenes or vinyl arenes is underdeveloped and challenging due to the low rotational barrier and weak configurational stability of such molecules. Therefore, the development of powerful strategies for the catalytic atroposelective synthesis of axially chiral styrenes or vinyl arenes is of great importance. In this work, we have accomplished the first atroposelective access to oxindole-based axially chiral styrenes by the strategy of catalytic kinetic resolution, and this strategy offered two kinds of oxindole-based axially chiral styrene derivatives in good diastereoselectivities (up to 94:6 dr) and excellent enantioselectivities (up to 98% ee) with high selectivity factors (S up to 106). This strategy not only provides easy access to oxindole-based axially chiral styrenes but also offers a robust method for synthesizing bisamide derivatives bearing both axial and central chirality. More importantly, this strategy has added a new class of members to the atropisomeric family, especially to the family of axially chiral styrenes.
中文翻译:

通过催化动力学拆分策略对基于羟吲哚的轴向手性苯乙烯的Atroposelective 访问
由于轴向手性分子的重要性,轴向手性分子的阻旋选择性合成引起了化学家的极大关注。然而,由于这些分子的低旋转势垒和弱构型稳定性,轴向手性苯乙烯或乙烯基芳烃的催化不对称合成不发达且具有挑战性。因此,开发用于轴向手性苯乙烯或乙烯基芳烃的催化阻转选择性合成的强大策略非常重要。在这项工作中,我们通过催化动力学拆分策略完成了首次对羟吲哚基轴向手性苯乙烯的阻转选择性访问,该策略提供了两种具有良好非对映选择性(高达94:6 dr)和出色的对映选择性(高达 98% ee)以及高选择性因子(S 高达 106)。这种策略不仅可以轻松获得基于羟吲哚的轴向手性苯乙烯,而且还为合成具有轴向和中心手性的双酰胺衍生物提供了一种可靠的方法。更重要的是,该策略为阻转异构家族,特别是轴向手性苯乙烯家族增加了一类新成员。
更新日期:2020-08-26
中文翻译:

通过催化动力学拆分策略对基于羟吲哚的轴向手性苯乙烯的Atroposelective 访问
由于轴向手性分子的重要性,轴向手性分子的阻旋选择性合成引起了化学家的极大关注。然而,由于这些分子的低旋转势垒和弱构型稳定性,轴向手性苯乙烯或乙烯基芳烃的催化不对称合成不发达且具有挑战性。因此,开发用于轴向手性苯乙烯或乙烯基芳烃的催化阻转选择性合成的强大策略非常重要。在这项工作中,我们通过催化动力学拆分策略完成了首次对羟吲哚基轴向手性苯乙烯的阻转选择性访问,该策略提供了两种具有良好非对映选择性(高达94:6 dr)和出色的对映选择性(高达 98% ee)以及高选择性因子(S 高达 106)。这种策略不仅可以轻松获得基于羟吲哚的轴向手性苯乙烯,而且还为合成具有轴向和中心手性的双酰胺衍生物提供了一种可靠的方法。更重要的是,该策略为阻转异构家族,特别是轴向手性苯乙烯家族增加了一类新成员。



























 京公网安备 11010802027423号
京公网安备 11010802027423号