当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
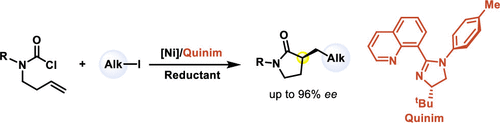
Quinim: a New Ligand Scaffold Enabled Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated γ-Lactam
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-26 , DOI: 10.1021/jacs.0c07126 Xianqing Wu 1 , Jingping Qu 1 , Yifeng Chen 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-26 , DOI: 10.1021/jacs.0c07126 Xianqing Wu 1 , Jingping Qu 1 , Yifeng Chen 1
Affiliation
|
Herein, we report a nickel-catalyzed reductive cross-coupling reaction of easily accessible 3-butenyl carbamoyl chloride with primary alkyl iodide to access the chiral α-alkylated pyrrolidinone with broad substrate scope and high ee. The current art of synthesis still remains challenging on the enantioselective α-monoalkylation of pyrrolidinones. The newly designed chiral 8-quinoline imidazoline ligand (Quinim) is crucial for maintaining the reactivity and enantioselectivity to ensure the reductive cyclization of mono-substituted alkenes for unprecedented synthesis of chiral nonaromatic heterocycles.
中文翻译:

Quinim:一种新的配体支架,使镍催化对映选择性合成 α-烷基化 γ-内酰胺
在此,我们报告了镍催化的 3-丁烯基氨基甲酰氯与伯烷基碘的还原交叉偶联反应,以获得具有广泛底物范围和高 ee 的手性 α-烷基化吡咯烷酮。目前的合成技术对吡咯烷酮的对映选择性 α-单烷基化仍然具有挑战性。新设计的手性 8-喹啉咪唑啉配体(Quinim)对于保持反应性和对映选择性至关重要,以确保单取代烯烃的还原环化,从而前所未有地合成手性非芳香杂环。
更新日期:2020-08-26
中文翻译:

Quinim:一种新的配体支架,使镍催化对映选择性合成 α-烷基化 γ-内酰胺
在此,我们报告了镍催化的 3-丁烯基氨基甲酰氯与伯烷基碘的还原交叉偶联反应,以获得具有广泛底物范围和高 ee 的手性 α-烷基化吡咯烷酮。目前的合成技术对吡咯烷酮的对映选择性 α-单烷基化仍然具有挑战性。新设计的手性 8-喹啉咪唑啉配体(Quinim)对于保持反应性和对映选择性至关重要,以确保单取代烯烃的还原环化,从而前所未有地合成手性非芳香杂环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号