当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chlorinated Byproduct Formation during the Electrochemical Advanced Oxidation Process at Magnéli Phase Ti4O7 Electrodes.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.est.0c03916 Meng-Hsuan Lin 1 , Devon Manley Bulman 2 , Christina K Remucal 2, 3 , Brian P Chaplin 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.est.0c03916 Meng-Hsuan Lin 1 , Devon Manley Bulman 2 , Christina K Remucal 2, 3 , Brian P Chaplin 1
Affiliation
|
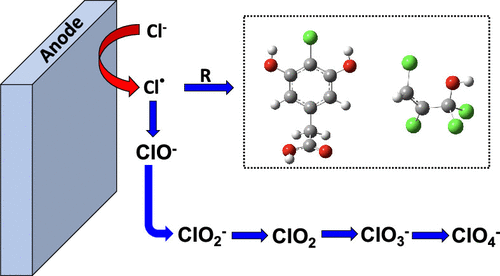
This research investigated chlorinated byproduct formation at Ti4O7 anodes. Resorcinol was used as a model organic compound representative of reactive phenolic groups in natural organic matter and industrial phenolic contaminants and was oxidized in the presence of NaCl (0—5 mM). Resorcinol mineralization was >68% in the presence and absence of NaCl at 3.1 V/SHE (residence time = 13 s). Results indicated that ∼4.3% of the initial chloride was converted to inorganic byproducts (free Cl2, ClO2–, ClO3–) in the absence of resorcinol, and this value decreased to <0.8% in the presence of resorcinol. Perchlorate formation rates from chlorate oxidation were 115—371 mol m–2 h–1, approximately two orders of magnitude lower than reported values for boron-doped diamond anodes. Liquid chromatography–mass spectroscopy detected two chlorinated organic products. Multichlorinated alcohol compounds (C3H2Cl4O and C3H4Cl4O) at 2.5 V/SHE and a monochlorinated phenolic compound (C8H7O4Cl) at 3.1 V/SHE were proposed as possible structures. Density functional theory calculations estimated that the proposed alcohol products were resistant to direct oxidation at 2.5 V/SHE, and the C8H7O4Cl compound was likely a transient intermediate. Chlorinated byproducts should be carefully monitored during electrochemical advanced oxidation processes, and multibarrier treatment approaches are likely necessary to prevent halogenated byproducts in the treated water.
中文翻译:

Magnéli相Ti4O7电极在电化学高级氧化过程中形成氯化副产物。
这项研究调查了在Ti 4 O 7阳极上氯化副产物的形成。间苯二酚被用作模型有机化合物,代表天然有机物和工业酚类污染物中的反应性酚基,并在NaCl(0-5 mM)存在下被氧化。在3.1 V / SHE的NaCl存在和不存在下,间苯二酚的矿化度> 68%(停留时间= 13 s)。结果表明,初始氯化~4.3%转化为无机副产物(游离氯2,CLO 2 -,CLO 3 - )在没有间苯二酚,并且该值在间苯二酚的存在减少到<0.8%。氯酸盐氧化产生的高氯酸盐形成速率为115-371 mol m –2h –1,比硼掺杂金刚石阳极的报告值低大约两个数量级。液相色谱-质谱法检测到两种氯化有机产物。提出了在2.5 V / SHE下的多氯醇化合物(C 3 H 2 Cl 4 O和C 3 H 4 Cl 4 O)和在3.1 V / SHE下的单氯酚化合物(C 8 H 7 O 4 Cl)作为可能的结构。密度泛函理论计算估计,所提出的醇产品在2.5 V / SHE和C 8 H 7 O 4下具有抗直接氧化的能力Cl化合物可能是瞬时中间体。在电化学高级氧化过程中,应仔细监测氯化副产物,并且可能需要采用多壁垒处理方法来防止处理后水中的卤化副产物。
更新日期:2020-10-06
中文翻译:

Magnéli相Ti4O7电极在电化学高级氧化过程中形成氯化副产物。
这项研究调查了在Ti 4 O 7阳极上氯化副产物的形成。间苯二酚被用作模型有机化合物,代表天然有机物和工业酚类污染物中的反应性酚基,并在NaCl(0-5 mM)存在下被氧化。在3.1 V / SHE的NaCl存在和不存在下,间苯二酚的矿化度> 68%(停留时间= 13 s)。结果表明,初始氯化~4.3%转化为无机副产物(游离氯2,CLO 2 -,CLO 3 - )在没有间苯二酚,并且该值在间苯二酚的存在减少到<0.8%。氯酸盐氧化产生的高氯酸盐形成速率为115-371 mol m –2h –1,比硼掺杂金刚石阳极的报告值低大约两个数量级。液相色谱-质谱法检测到两种氯化有机产物。提出了在2.5 V / SHE下的多氯醇化合物(C 3 H 2 Cl 4 O和C 3 H 4 Cl 4 O)和在3.1 V / SHE下的单氯酚化合物(C 8 H 7 O 4 Cl)作为可能的结构。密度泛函理论计算估计,所提出的醇产品在2.5 V / SHE和C 8 H 7 O 4下具有抗直接氧化的能力Cl化合物可能是瞬时中间体。在电化学高级氧化过程中,应仔细监测氯化副产物,并且可能需要采用多壁垒处理方法来防止处理后水中的卤化副产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号