当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Chirality on the Compression of 2-(2-Oxo-1-pyrrolidinyl)butyramide: A Tale of Two Crystals
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.cgd.0c00871 Suse S. Bebiano 1, 2 , Joop H. ter Horst 1, 2 , Iain D.H. Oswald 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.cgd.0c00871 Suse S. Bebiano 1, 2 , Joop H. ter Horst 1, 2 , Iain D.H. Oswald 1
Affiliation
|
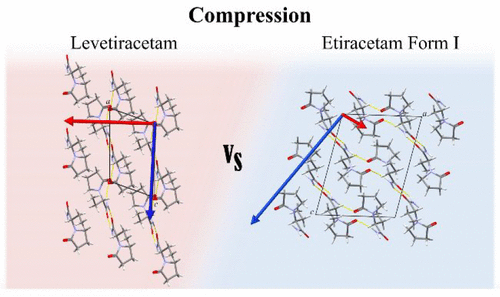
Understanding polymorphism in chiral systems for drug manufacturing is essential to avoid undesired therapeutic effects. Generally, polymorphism is studied through changes in temperature and solution concentration. A less common approach is the application of pressure. The goal of this work is to investigate the effect of pressure on levetiracetam (pure enantiomer) and etiracetam (racemic compound). Anisotropic compressions of levetiracetam and etiracetam are observed to 5.26 and 6.29 GPa, respectively. The most compressible direction for both was identified to be perpendicular to the layers of the structure. Raman spectroscopy and an analysis of intermolecular interactions suggest subtle phase transitions in levetiracetam (∼2 GPa) and etiracetam (∼1.5 GPa). The stability of etiracetam increases with respect to levetiracetam on compression; hence, the chiral resolution of this system is unfavorable using pressure. This work contributes to the ongoing efforts in understanding the stability of chiral systems.
中文翻译:

手性对2-(2-氧-1-吡咯烷基)丁酰胺压缩的影响:两个晶体的故事
了解手性系统中用于药物制造的多态性对于避免不良的治疗效果至关重要。通常,通过温度和溶液浓度的变化研究多态性。不太常见的方法是施加压力。这项工作的目的是研究压力对左乙拉西坦(纯对映体)和乙拉西坦(外消旋化合物)的影响。左乙拉西坦和乙拉西坦的各向异性压缩分别观察到5.26和6.29 GPa。两者的最可压缩方向被确定为垂直于结构层。拉曼光谱法和分子间相互作用的分析表明左乙拉西坦(〜2 GPa)和乙拉西坦(〜1.5 GPa)存在细微的相变。依拉西坦的压缩性相对于左乙拉西坦增加。因此,使用压力不利于该系统的手性拆分。这项工作有助于不断努力了解手性系统的稳定性。
更新日期:2020-10-07
中文翻译:

手性对2-(2-氧-1-吡咯烷基)丁酰胺压缩的影响:两个晶体的故事
了解手性系统中用于药物制造的多态性对于避免不良的治疗效果至关重要。通常,通过温度和溶液浓度的变化研究多态性。不太常见的方法是施加压力。这项工作的目的是研究压力对左乙拉西坦(纯对映体)和乙拉西坦(外消旋化合物)的影响。左乙拉西坦和乙拉西坦的各向异性压缩分别观察到5.26和6.29 GPa。两者的最可压缩方向被确定为垂直于结构层。拉曼光谱法和分子间相互作用的分析表明左乙拉西坦(〜2 GPa)和乙拉西坦(〜1.5 GPa)存在细微的相变。依拉西坦的压缩性相对于左乙拉西坦增加。因此,使用压力不利于该系统的手性拆分。这项工作有助于不断努力了解手性系统的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号