当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereoselective Trapping of Transient Carboxylic Oxonium Ylides with α,β‐Unsaturated 2‐Acyl Imidazoles
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000701 Mengchu Zhang 1 , Tianyuan Zhang 1 , Dan Zhang 1 , Wenhao Hu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000701 Mengchu Zhang 1 , Tianyuan Zhang 1 , Dan Zhang 1 , Wenhao Hu 1
Affiliation
|
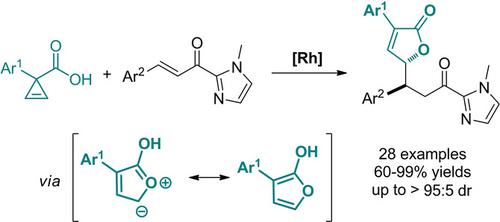
By developing a diastereoselective reaction of cyclopropene carboxylic acids with α,β‐unsaturated 2‐acyl imidazoles, we reported here a Michael‐type trapping of transient carboxylic oxonium ylides. This transformation provides a direct approach for the construction of valuable γ‐butenolide derivatives in good yields (60–99%) with high diastereoselectivities (up to >95:5 dr) under mild reaction conditions.
中文翻译:

α,β-不饱和2-酰基咪唑类对瞬态羧酸氧鎓盐的非对映选择性捕获
通过开发环丙烯羧酸与α,β-不饱和2-酰基咪唑的非对映选择性反应,我们在此报道了瞬态羧酸氧鎓叶立德的Michael型捕集。这种转化为在温和的反应条件下以高非对映选择性(高达> 95:5 dr)以高收率(60–99%)构建有价值的γ-丁烯内酯衍生物提供了一种直接方法。
更新日期:2020-08-25
中文翻译:

α,β-不饱和2-酰基咪唑类对瞬态羧酸氧鎓盐的非对映选择性捕获
通过开发环丙烯羧酸与α,β-不饱和2-酰基咪唑的非对映选择性反应,我们在此报道了瞬态羧酸氧鎓叶立德的Michael型捕集。这种转化为在温和的反应条件下以高非对映选择性(高达> 95:5 dr)以高收率(60–99%)构建有价值的γ-丁烯内酯衍生物提供了一种直接方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号