当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Decahydrocyclobuta[cd]indene Skeletons: Rhodium(III)‐Catalyzed Hydroarylation and Relay Thiophene‐Promoted Intramolecular [2+2] Cycloaddition
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000825 Dingding Gao 1 , Feng Wang 1 , Xing‐Yu Liu 2 , Kai‐Rui Feng 1 , Jia‐Ying Zhao 1 , Yu‐Hui Wang 1 , Xiao‐Di Yang 1 , Ping Tian 1, 2, 3 , Guo‐Qiang Lin 1, 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000825 Dingding Gao 1 , Feng Wang 1 , Xing‐Yu Liu 2 , Kai‐Rui Feng 1 , Jia‐Ying Zhao 1 , Yu‐Hui Wang 1 , Xiao‐Di Yang 1 , Ping Tian 1, 2, 3 , Guo‐Qiang Lin 1, 2
Affiliation
|
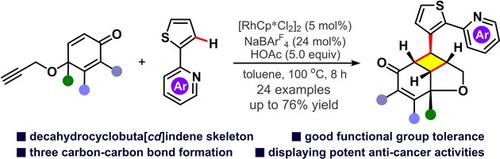
The preparation of decahydrocyclobuta[cd]indene skeleton was accomplished through rhodium(III)‐catalyzed hydroarylation and relay thiophene‐promoted intramolecular [2+2] cycloaddition. This tandem reaction exhibited broad substrate scope (24 examples) and good functional group compatibility. Control experiments revealed the important role of sulfur (S) heteroatom, thus a tentative mechanism with thiophene‐promoted double Michael additions was proposed to explain this formal [2+2] cycloaddition. Moreover, the resulting polycyclic products displayed potent anti‐cancer activities against breast cancer cell lines MDA‐MB‐468.
中文翻译:

十氢环丁基[cd]茚骨架的合成:铑(III)催化的氢芳基化和噻吩促进的分子内[2 + 2]环加成反应
十氢环丁[ cd ]茚骨架的制备是通过铑(III)催化的氢芳基化反应和噻吩促进的分子内[2 + 2]环加成反应完成的。该串联反应表现出广泛的底物范围(24个实施例)和良好的官能团相容性。对照实验揭示了硫(S)杂原子的重要作用,因此提出了一种由噻吩促进的双迈克尔加成的尝试性机理来解释这种正式的[2 + 2]环加成。此外,所得的多环产物对乳腺癌细胞系MDA-MB-468表现出有效的抗癌活性。
更新日期:2020-10-26
中文翻译:

十氢环丁基[cd]茚骨架的合成:铑(III)催化的氢芳基化和噻吩促进的分子内[2 + 2]环加成反应
十氢环丁[ cd ]茚骨架的制备是通过铑(III)催化的氢芳基化反应和噻吩促进的分子内[2 + 2]环加成反应完成的。该串联反应表现出广泛的底物范围(24个实施例)和良好的官能团相容性。对照实验揭示了硫(S)杂原子的重要作用,因此提出了一种由噻吩促进的双迈克尔加成的尝试性机理来解释这种正式的[2 + 2]环加成。此外,所得的多环产物对乳腺癌细胞系MDA-MB-468表现出有效的抗癌活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号