Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.tetlet.2020.152321 Sudhakar Kalagara , Gabriel Orozco , Shizue Mito
|
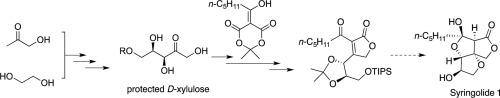
Wittig reaction and asymmetric dihydroxylation were used as the key steps in the synthesis of d-xylulose, a commercially available but costly carbohydrate. The effects of protecting groups and reactions conditions on asymmetric dihydroxylation are demonstrated. Optically pure d-xylulose was obtained after 4–6 steps from readily available hydroxyacetone and ethylene glycol. The method also involves some other valuable intermediates along the synthesis. Those intermediates were applied in the formal synthesis of Syringolides. A key precursor butenolide to Syringolide 1, the first non-proteinaceous specific elicitors of plant hypersensitive response, was obtained after 3 steps from the intermediate (8–10 steps from hydroxyacetone and ethylene glycol).
中文翻译:

d-木酮糖的有效合成和丁香三醇一的形式合成
Wittig反应和不对称二羟基化被用作合成d-木酮糖的关键步骤,d-木酮糖是可商购但昂贵的碳水化合物。证明了保护基和反应条件对不对称二羟基化的影响。经过4–6个步骤,从容易获得的羟基丙酮和乙二醇中获得了光学纯的D-木酮糖。该方法在合成过程中还涉及其他一些有价值的中间体。这些中间体被用于丁香酚的形式合成。从中间产物经过3步(从羟丙酮和乙二醇到8-10步)后,获得了丁香内酯1的关键前体丁烯内酯,这是植物超敏反应的第一个非蛋白质特异性引发剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号