当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Streamlined targeting of Amaryllidaceae alkaloids from the bulbs of Crinum scillifolium using spectrometric and taxonomically-informed scoring metabolite annotations
Phytochemistry ( IF 3.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.phytochem.2020.112485 Amon Diane N'Tamon 1 , Aboua Timothée Okpekon 2 , Nicaise F Bony 3 , Guillaume Bernadat 4 , Jean-François Gallard 5 , Tapé Kouamé 6 , Blandine Séon-Méniel 4 , Karine Leblanc 4 , Somia Rharrabti 4 , Elisabeth Mouray 7 , Philippe Grellier 7 , Michèle Ake 3 , N'Cho Christophe Amin 3 , Pierre Champy 4 , Mehdi A Beniddir 4 , Pierre Le Pogam 4
Phytochemistry ( IF 3.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.phytochem.2020.112485 Amon Diane N'Tamon 1 , Aboua Timothée Okpekon 2 , Nicaise F Bony 3 , Guillaume Bernadat 4 , Jean-François Gallard 5 , Tapé Kouamé 6 , Blandine Séon-Méniel 4 , Karine Leblanc 4 , Somia Rharrabti 4 , Elisabeth Mouray 7 , Philippe Grellier 7 , Michèle Ake 3 , N'Cho Christophe Amin 3 , Pierre Champy 4 , Mehdi A Beniddir 4 , Pierre Le Pogam 4
Affiliation
|
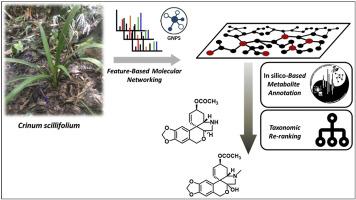
Four undescribed alkaloids have been isolated from the bulbs of the previously unstudied Crinum scillifolium. These compounds were targeted following a state-of-the-art molecular networking strategy comprising a dereplication against in silico databases and re-ranking of the candidate structures based on taxonomically informed scoring. The unreported structures span across a variety of Amaryllidaceae alkaloids appendages. Their structures were unambiguously elucidated by thorough interpretation of their HRESIMS and 1D and 2D NMR data, and comparison to literature data. DFT-NMR calculations were performed to support the determined relative configurations of scillitazettine and scilli-N-desmethylpretazettine and their absolute configurations were mitigated by comparison between experimental and theoretically calculated ECD spectra. The lack of a methyl group on the nitrogen atom in the structure of scilli-N-desmethylpretazettine series is highly unusual in the pretazettine/tazettine series but the most original structural feature in it lies in its 11α disposed hydrogen, which is new to pretazettines. The antiplasmodial as well as the cytotoxic activities against the human colon cancer cell line HCT116 were evaluated, revealing mild to null activities.
中文翻译:

使用光谱和分类学信息的评分代谢物注释简化了从 Crinum scillifolium 球茎中提取石蒜科生物碱的过程
四种未描述的生物碱已从先前未研究的 Crinum scillifolium 的球茎中分离出来。这些化合物的目标是遵循最先进的分子网络策略,包括对计算机数据库的去复制和基于分类学信息的评分对候选结构进行重新排序。未报告的结构跨越各种石蒜科生物碱附属物。通过对 HRESIMS 和 1D 和 2D NMR 数据的彻底解释,以及与文献数据的比较,明确阐明了它们的结构。进行 DFT-NMR 计算以支持确定的 scillitazettine 和 scilli-N-desmethylpretazettine 的相对构型,并且通过比较实验和理论计算的 ECD 光谱来减轻它们的绝对构型。scilli-N-去甲基pretazettine系列结构中氮原子上缺少甲基基团在pretazettine/tazettine系列中非常罕见,但其中最原始的结构特征在于其11α位氢,这对pretazettine来说是新的。评估了抗疟原虫以及对人结肠癌细胞系 HCT116 的细胞毒活性,揭示了轻度至无效的活性。
更新日期:2020-11-01
中文翻译:

使用光谱和分类学信息的评分代谢物注释简化了从 Crinum scillifolium 球茎中提取石蒜科生物碱的过程
四种未描述的生物碱已从先前未研究的 Crinum scillifolium 的球茎中分离出来。这些化合物的目标是遵循最先进的分子网络策略,包括对计算机数据库的去复制和基于分类学信息的评分对候选结构进行重新排序。未报告的结构跨越各种石蒜科生物碱附属物。通过对 HRESIMS 和 1D 和 2D NMR 数据的彻底解释,以及与文献数据的比较,明确阐明了它们的结构。进行 DFT-NMR 计算以支持确定的 scillitazettine 和 scilli-N-desmethylpretazettine 的相对构型,并且通过比较实验和理论计算的 ECD 光谱来减轻它们的绝对构型。scilli-N-去甲基pretazettine系列结构中氮原子上缺少甲基基团在pretazettine/tazettine系列中非常罕见,但其中最原始的结构特征在于其11α位氢,这对pretazettine来说是新的。评估了抗疟原虫以及对人结肠癌细胞系 HCT116 的细胞毒活性,揭示了轻度至无效的活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号