当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Cyclopropanes via Activation of (γ-Methoxy)alkyl Gold(I) Complexes with Lewis Acids
Organometallics ( IF 2.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.organomet.0c00324 Nana Kim 1 , Ross A. Widenhoefer 1
Organometallics ( IF 2.8 ) Pub Date : 2020-08-24 , DOI: 10.1021/acs.organomet.0c00324 Nana Kim 1 , Ross A. Widenhoefer 1
Affiliation
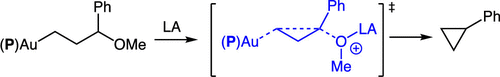
|
Treatment of the gold 3-methoxy-3-phenylpropyl complex (P)AuCH2CH2CH(OMe)Ph [P = P(t-Bu)2o-biphenyl] with AlCl3 at −78 °C led to the immediate (≤5 min) formation of a 4:1 mixture of phenylcyclopropane and (1-methoxypropyl)benzene in 86 ± 5% combined yield. Lewis acid activation of the stereochemically pure isotopomer erythro-(P)AuCH2CHDCH(OMe)Ph led to the formation of cis-2-deuterio-1-phenylcyclopropane in 84 ± 5% yield as a single stereoisomer, which established that cyclopropanation occurred with inversion of the γ-stereocenter. Similarly, ionization of the stereochemically pure cyclohexyl gold complex cis-(P)Au CHCH2CH(OMe)CH2CH2
CHCH2CH(OMe)CH2CH2 CH2 at −78 °C formed bicyclo[3.1.0]hexane in 82% ± 5% yield, which validated a low energy pathway for cyclopropanation involving inversion of the α-stereocenter. Taken together, these observations are consistent with a mechanism for cyclopropane formation involving backside displacement of both the Cγ leaving group and the Cα (L)Au+ fragment via a W-shaped transition state.
CH2 at −78 °C formed bicyclo[3.1.0]hexane in 82% ± 5% yield, which validated a low energy pathway for cyclopropanation involving inversion of the α-stereocenter. Taken together, these observations are consistent with a mechanism for cyclopropane formation involving backside displacement of both the Cγ leaving group and the Cα (L)Au+ fragment via a W-shaped transition state.
中文翻译:

通过路易斯酸活化(γ-甲氧基)烷基金(I)配合物形成环丙烷
在−78°C下用AlCl 3处理3-甲氧基-3-苯基丙基金(P)AuCH 2 CH 2 CH(OMe)Ph [ P = P(t -Bu)2 o-联苯]金导致立即(≤5分钟)形成苯基环丙烷和(1-甲氧基丙基)苯的4:1混合物,合并产率为86±5%。立体化学纯的异构体赤型-(P)AuCH 2 CHDCH(OMe)Ph的路易斯酸活化导致顺式形成-2-氘代-1-苯基环丙烷为单一立体异构体,产率为84±5%,这表明环丙烷化是随着γ-立体中心的反转而发生的。同样,立体化学纯的环己基金配合物顺式-(P)Au CHCH 2 CH(OMe)CH 2 CH 2
CHCH 2 CH(OMe)CH 2 CH 2 CH 2在-78°C下电离形成双环[3.1.0]己烷,产率为82%±5%,验证了涉及α-立体中心反转的环丙烷化低能途径。综上所述,这些观察结果与环丙烷形成机理一致,该机理涉及Cγ离去基团和Cα(L)Au +片段通过W形过渡态向后位移。
CH 2在-78°C下电离形成双环[3.1.0]己烷,产率为82%±5%,验证了涉及α-立体中心反转的环丙烷化低能途径。综上所述,这些观察结果与环丙烷形成机理一致,该机理涉及Cγ离去基团和Cα(L)Au +片段通过W形过渡态向后位移。
更新日期:2020-09-14
 CHCH2CH(OMe)CH2CH2
CHCH2CH(OMe)CH2CH2 CH2 at −78 °C formed bicyclo[3.1.0]hexane in 82% ± 5% yield, which validated a low energy pathway for cyclopropanation involving inversion of the α-stereocenter. Taken together, these observations are consistent with a mechanism for cyclopropane formation involving backside displacement of both the Cγ leaving group and the Cα (L)Au+ fragment via a W-shaped transition state.
CH2 at −78 °C formed bicyclo[3.1.0]hexane in 82% ± 5% yield, which validated a low energy pathway for cyclopropanation involving inversion of the α-stereocenter. Taken together, these observations are consistent with a mechanism for cyclopropane formation involving backside displacement of both the Cγ leaving group and the Cα (L)Au+ fragment via a W-shaped transition state.
中文翻译:

通过路易斯酸活化(γ-甲氧基)烷基金(I)配合物形成环丙烷
在−78°C下用AlCl 3处理3-甲氧基-3-苯基丙基金(P)AuCH 2 CH 2 CH(OMe)Ph [ P = P(t -Bu)2 o-联苯]金导致立即(≤5分钟)形成苯基环丙烷和(1-甲氧基丙基)苯的4:1混合物,合并产率为86±5%。立体化学纯的异构体赤型-(P)AuCH 2 CHDCH(OMe)Ph的路易斯酸活化导致顺式形成-2-氘代-1-苯基环丙烷为单一立体异构体,产率为84±5%,这表明环丙烷化是随着γ-立体中心的反转而发生的。同样,立体化学纯的环己基金配合物顺式-(P)Au
 CHCH 2 CH(OMe)CH 2 CH 2
CHCH 2 CH(OMe)CH 2 CH 2 CH 2在-78°C下电离形成双环[3.1.0]己烷,产率为82%±5%,验证了涉及α-立体中心反转的环丙烷化低能途径。综上所述,这些观察结果与环丙烷形成机理一致,该机理涉及Cγ离去基团和Cα(L)Au +片段通过W形过渡态向后位移。
CH 2在-78°C下电离形成双环[3.1.0]己烷,产率为82%±5%,验证了涉及α-立体中心反转的环丙烷化低能途径。综上所述,这些观察结果与环丙烷形成机理一致,该机理涉及Cγ离去基团和Cα(L)Au +片段通过W形过渡态向后位移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号