当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extensive Plasmid Library to Prepare Tau Protein Variants and Study Their Functional Biochemistry.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-08-24 , DOI: 10.1021/acschemneuro.0c00469 Thomas K Karikari 1, 2, 3 , Sophie Keeling 1 , Emily Hill 1 , Juan Lantero Rodrı Guez 3 , David A Nagel 4 , Bruno Becker 3, 5 , Kina Höglund 3, 5 , Henrik Zetterberg 3, 5, 6, 7 , Kaj Blennow 3, 5 , Eric J Hill 4 , Kevin G Moffat 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-08-24 , DOI: 10.1021/acschemneuro.0c00469 Thomas K Karikari 1, 2, 3 , Sophie Keeling 1 , Emily Hill 1 , Juan Lantero Rodrı Guez 3 , David A Nagel 4 , Bruno Becker 3, 5 , Kina Höglund 3, 5 , Henrik Zetterberg 3, 5, 6, 7 , Kaj Blennow 3, 5 , Eric J Hill 4 , Kevin G Moffat 1
Affiliation
|
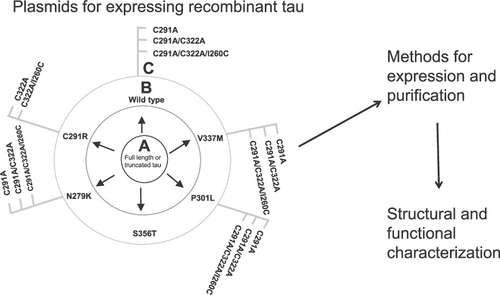
Tau neurofibrillary tangles are key pathological features of Alzheimer’s disease and other tauopathies. Recombinant protein technology is vital for studying the structure and function of tau in physiology and aggregation in pathophysiology. However, open-source and well-characterized plasmids for efficiently expressing and purifying different tau variants are lacking. We generated 44 sequence-verified plasmids including those encoding full length (FL) tau-441, its four-repeat microtubule-binding (K18) fragment, and their respective selected familial pathological variants (N279K, V337M, P301L, C291R, and S356T). Moreover, plasmids for expressing single (C291A), double (C291A/C322A), and triple (C291A/C322A/I260C) cysteine-modified variants were generated to study alterations in cysteine content and locations. Furthermore, protocols for producing representative tau forms were developed. We produced and characterized the aggregation behavior of the triple cysteine-modified tau-K18, often used in real-time cell internalization and aggregation studies because it can be fluorescently labeled on a cysteine outside the microtubule-binding core. Similar to the wild type (WT), triple cysteine-modified tau-K18 aggregated by progressive β-sheet enrichment, albeit at a slower rate. On prolonged incubation, cysteine-modified K18 formed paired helical filaments similar to those in Alzheimer’s disease, sharing morphological phenotypes with WT tau-K18 filaments. Nonetheless, cysteine-modified tau-K18 filaments were significantly shorter (p = 0.002) and mostly wider than WT filaments, explainable by their different principal filament elongation pathways: vertical (end-to-end) and lateral growth for WT and cysteine-modified, respectively. Cysteine rearrangement may therefore induce filament polymorphism. Together, the plasmid library, the protein production methods, and the new insights into cysteine-dependent aggregation should facilitate further studies and the design of antiaggregation agents.
中文翻译:

广泛的质粒文库,用于制备Tau蛋白变体并研究其功能生化。
Tau神经原纤维缠结是阿尔茨海默氏病和其他陶氏病的关键病理特征。重组蛋白技术对于研究tau在生理学中的结构和功能以及在病理生理学中的聚集至关重要。但是,缺乏用于有效表达和纯化不同tau变体的开源和特征明确的质粒。我们生成了44个经过序列验证的质粒,包括编码全长(FL)tau-441,其四重复微管结合(K18)片段的质粒,以及它们各自选择的家族病理变异(N279K,V337M,P301L,C291R和S356T) 。此外,产生了表达单个(C291A),双重(C291A / C322A)和三次(C291A / C322A / I260C)半胱氨酸修饰的变体的质粒,以研究半胱氨酸含量和位置的变化。此外,制定了生产代表性tau表格的协议。我们产生并表征了三倍半胱氨酸修饰的tau-K18的聚集行为,该聚集行为经常用于实时细胞内在化和聚集研究,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(通常在实时细胞内化和聚集研究中使用,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(通常在实时细胞内化和聚集研究中使用,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(p = 0.002),并且比WT细丝更宽,这可以用它们不同的主要细丝伸长途径来解释:WT和半胱氨酸修饰的分别垂直(端到端)和侧向生长。因此,半胱氨酸重排可能引起细丝多态性。总之,质粒文库,蛋白质生产方法以及对半胱氨酸依赖性聚集的新见解应有助于进一步研究和设计抗聚集剂。
更新日期:2020-10-07
中文翻译:

广泛的质粒文库,用于制备Tau蛋白变体并研究其功能生化。
Tau神经原纤维缠结是阿尔茨海默氏病和其他陶氏病的关键病理特征。重组蛋白技术对于研究tau在生理学中的结构和功能以及在病理生理学中的聚集至关重要。但是,缺乏用于有效表达和纯化不同tau变体的开源和特征明确的质粒。我们生成了44个经过序列验证的质粒,包括编码全长(FL)tau-441,其四重复微管结合(K18)片段的质粒,以及它们各自选择的家族病理变异(N279K,V337M,P301L,C291R和S356T) 。此外,产生了表达单个(C291A),双重(C291A / C322A)和三次(C291A / C322A / I260C)半胱氨酸修饰的变体的质粒,以研究半胱氨酸含量和位置的变化。此外,制定了生产代表性tau表格的协议。我们产生并表征了三倍半胱氨酸修饰的tau-K18的聚集行为,该聚集行为经常用于实时细胞内在化和聚集研究,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(通常在实时细胞内化和聚集研究中使用,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(通常在实时细胞内化和聚集研究中使用,因为它可以在微管结合核心外部的半胱氨酸上进行荧光标记。与野生型(WT)相似,三聚半胱氨酸修饰的tau-K18通过渐进的β-折叠富集而聚集,尽管速度较慢。长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(长时间孵育后,半胱氨酸修饰的K18形成与阿尔茨海默氏病相似的成对螺旋丝,与WT tau-K18丝共享形态表型。尽管如此,半胱氨酸修饰的tau-K18细丝却明显较短(p = 0.002),并且比WT细丝更宽,这可以用它们不同的主要细丝伸长途径来解释:WT和半胱氨酸修饰的分别垂直(端到端)和侧向生长。因此,半胱氨酸重排可能引起细丝多态性。总之,质粒文库,蛋白质生产方法以及对半胱氨酸依赖性聚集的新见解应有助于进一步研究和设计抗聚集剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号