当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogen Bonding-Mediated Enhancement of Bioinspired Electrochemical Nitrogen Reduction on Cu2–xS Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-08-24 , DOI: 10.1021/acscatal.0c01730 Min-Cheol Kim 1 , Hyunji Nam 2, 3 , Jihyun Choi 4, 5 , Hee Soo Kim 4 , Hong Woo Lee 1, 3 , Donghun Kim 1 , Jimin Kong 4, 5 , Sang Soo Han 1 , Seung Yong Lee 2 , Hyun S. Park 4
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-08-24 , DOI: 10.1021/acscatal.0c01730 Min-Cheol Kim 1 , Hyunji Nam 2, 3 , Jihyun Choi 4, 5 , Hee Soo Kim 4 , Hong Woo Lee 1, 3 , Donghun Kim 1 , Jimin Kong 4, 5 , Sang Soo Han 1 , Seung Yong Lee 2 , Hyun S. Park 4
Affiliation
|
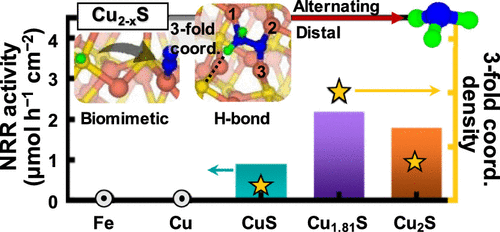
The electrochemical nitrogen reduction reaction (NRR) has been regarded as a promising alternative to the conventional Haber–Bosh process for NH3 synthesis. Inspired by the Fe–Mo–S cofactor in the enzyme nitrogenase, metal sulfide catalysts, mostly Fe- or Mo-based sulfides, have recently received great interest. Here, we propose Cu2–xS (0 ≤ x < 1) as an efficient NRR electrocatalyst. Electrochemical tests at room temperature and atmospheric pressure reveal that Cu1.81S achieves a high NH3 yield of 2.19 μmol h–1 cm–2 along with a Faradaic efficiency of 14.1% at −0.1 V versus reversible hydrogen electrode in an aqueous electrolyte. According to our first-principles calculations, the superior NRR properties originate from the threefold-coordinate Cu sites in Cu1.81S. These sites enable N–H···S hydrogen bonding during N2Hy adsorption and stabilize the intermediates on the Cu2–xS surfaces, leading to a significant decrease in the overpotential limit for the NRR. Moreover, the threefold sites not only provide a bioinspired NRR pathway similar to that of nitrogenase but also enable both distal and alternating pathways, increasing the efficiency for the NRR. This study presents an attractive electrocatalyst for the NRR and opens an alternative route to explore chalcogenides and halides as NRR catalysts where the hydrogen-bonding mediation can similarly operate.
中文翻译:

氢键介导的Cu 2– x S催化剂上生物启发的电化学氮还原的增强
电化学氮还原反应(NRR)已被认为是用于合成NH 3的常规Haber-Bosh工艺的有希望的替代方法。受金属酶硫化物中Fe–Mo–S辅助因子的启发,金属硫化物催化剂(主要是基于Fe或Mo的硫化物)最近引起了人们的极大兴趣。在这里,我们提出将Cu 2- x S(0≤x <1)作为一种有效的NRR电催化剂。在室温和大气压下的电化学测试表明,Cu 1.81 S实现了2.19μmolh –1 cm –2的高NH 3收率。相对于可逆氢电极在水性电解液中,在-0.1 V时的法拉第效率为14.1%。根据我们的第一性原理计算,优异的NRR特性来自于Cu 1.81 S中三重配位的Cu位。这些位使N–H···S在N 2 H y吸附过程中氢键结合,并使中间体稳定在Cu上2 – xS表面,导致NRR的超电势极限大大降低。此外,三重位点不仅提供了与固氮酶类似的生物启发性NRR途径,而且还启用了远端途径和交替途径,从而提高了NRR的效率。这项研究提出了一种有吸引力的NRR电催化剂,并开辟了一条探索硫族化物和卤化物作为NRR催化剂的替代途径,其中氢键介导可以类似地起作用。
更新日期:2020-09-20
中文翻译:

氢键介导的Cu 2– x S催化剂上生物启发的电化学氮还原的增强
电化学氮还原反应(NRR)已被认为是用于合成NH 3的常规Haber-Bosh工艺的有希望的替代方法。受金属酶硫化物中Fe–Mo–S辅助因子的启发,金属硫化物催化剂(主要是基于Fe或Mo的硫化物)最近引起了人们的极大兴趣。在这里,我们提出将Cu 2- x S(0≤x <1)作为一种有效的NRR电催化剂。在室温和大气压下的电化学测试表明,Cu 1.81 S实现了2.19μmolh –1 cm –2的高NH 3收率。相对于可逆氢电极在水性电解液中,在-0.1 V时的法拉第效率为14.1%。根据我们的第一性原理计算,优异的NRR特性来自于Cu 1.81 S中三重配位的Cu位。这些位使N–H···S在N 2 H y吸附过程中氢键结合,并使中间体稳定在Cu上2 – xS表面,导致NRR的超电势极限大大降低。此外,三重位点不仅提供了与固氮酶类似的生物启发性NRR途径,而且还启用了远端途径和交替途径,从而提高了NRR的效率。这项研究提出了一种有吸引力的NRR电催化剂,并开辟了一条探索硫族化物和卤化物作为NRR催化剂的替代途径,其中氢键介导可以类似地起作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号