当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temperature‐dependent kinetics of the simplest Criegee intermediate reaction with dimethyl sulfoxide
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-08-25 , DOI: 10.1002/jccs.202000206 Yu‐Lin Li, Chun‐Yu Lin, Yen‐Hsiu Lin, Jim Jr‐Min Lin
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-08-25 , DOI: 10.1002/jccs.202000206 Yu‐Lin Li, Chun‐Yu Lin, Yen‐Hsiu Lin, Jim Jr‐Min Lin
|
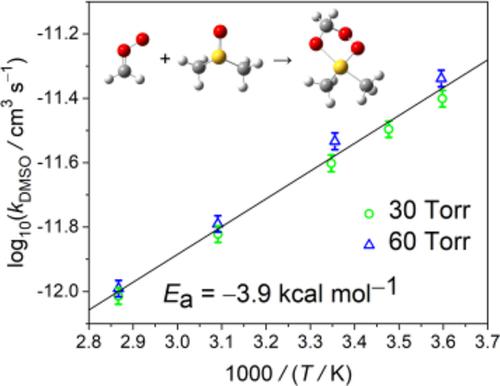
Criegee intermediates are thought to play roles in atmospheric chemistry, including OH radical formation, oxidation of SO2, NO2, etc. CH2OO is the simplest Criegee intermediate, of which the reactivity has been a hot topic. Here we investigated the kinetics of CH2OO reaction with dimethyl sulfoxide (DMSO) under 278–349 K and 10–150 Torr. DMSO is an important species formed in the oxidation of dimethyl sulfide in the biogenic sulfur cycle. The concentration of CH2OO was monitored in real‐time via its mid‐infrared absorption band at about 1,286 cm−1 (Q branch of the ν4 band) with a high‐resolution quantum cascade laser spectrometer. The 298 K bimolecular rate coefficient was determined to be k298 = (2.3 ± 0.3) × 10−12 cm3/s at 30 Torr with an Arrhenius activation energy of −3.9 ± 0.2 kcal/mol and a weak pressure dependence for pressures higher than 30 Torr (k298 = (2.8 ± 0.3) × 10−12 cm3/s at 100 Torr). The reaction is speculated to undergo a five‐membered ring intermediate, analogous to that of CH2OO with SO2. The negative activation energy indicates that the rate‐determining transition state is submerged. The magnitude of the reaction rate coefficient lies in between those of CH2OO reactions with (CH3)2CO and with SO2.
中文翻译:

最简单的Criegee中间体与二甲亚砜的温度依赖性动力学
Criegee中间体被认为在大气化学中起作用,包括OH自由基的形成,SO 2,NO 2等的氧化。CH 2 OO是最简单的Criegee中间体,其反应性一直是热门话题。在这里,我们研究了在278–349 K和10–150 Torr下CH 2 OO与二甲亚砜(DMSO)反应的动力学。DMSO是生物成因硫循环中二甲基硫醚氧化过程中形成的重要物种。CH的浓度2 OO在实时经由其中红外吸收带在约1286厘米监测-1(的Q分支ν 4波段)和高分辨率量子级联激光光谱仪。298 K双分子速率系数经确定为在30 Torr时为k 298 =(2.3±0.3)×10 -12 cm 3 / s,Arrhenius活化能为-3.9±0.2 kcal / mol,对较高压力的压力依赖性较弱。大于30托(100托时k 298 =(2.8±0.3)×10 -12 cm 3 / s)。推测该反应会经历五元环中间体,类似于CH 2 OO与SO 2的反应。负激活能表示确定速率的过渡状态已被淹没。反应速率系数的大小介于与(CH 3)2 CO和SO 2进行的CH 2 OO反应之间。
更新日期:2020-09-24
中文翻译:

最简单的Criegee中间体与二甲亚砜的温度依赖性动力学
Criegee中间体被认为在大气化学中起作用,包括OH自由基的形成,SO 2,NO 2等的氧化。CH 2 OO是最简单的Criegee中间体,其反应性一直是热门话题。在这里,我们研究了在278–349 K和10–150 Torr下CH 2 OO与二甲亚砜(DMSO)反应的动力学。DMSO是生物成因硫循环中二甲基硫醚氧化过程中形成的重要物种。CH的浓度2 OO在实时经由其中红外吸收带在约1286厘米监测-1(的Q分支ν 4波段)和高分辨率量子级联激光光谱仪。298 K双分子速率系数经确定为在30 Torr时为k 298 =(2.3±0.3)×10 -12 cm 3 / s,Arrhenius活化能为-3.9±0.2 kcal / mol,对较高压力的压力依赖性较弱。大于30托(100托时k 298 =(2.8±0.3)×10 -12 cm 3 / s)。推测该反应会经历五元环中间体,类似于CH 2 OO与SO 2的反应。负激活能表示确定速率的过渡状态已被淹没。反应速率系数的大小介于与(CH 3)2 CO和SO 2进行的CH 2 OO反应之间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号