Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-08-25 , DOI: 10.1016/j.yjmcc.2020.08.006 Janet R Manning 1 , Aruna B Wijeratne 2 , Brian B Oloizia 1 , Yu Zhang 1 , Kenneth D Greis 2 , Jo El J Schultz 1
|
Rationale
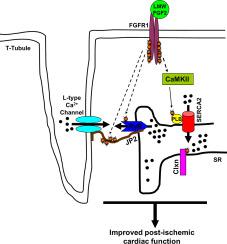
Among its many biological roles, fibroblast growth factor 2 (FGF2) protects the heart from dysfunction and damage associated with an ischemic attack. Our laboratory demonstrated that its protection against myocardial dysfunction occurs by the low molecular weight (LMW) isoform of FGF2, while the high molecular weight (HMW) isoforms are associated with a worsening in post-ischemic recovery of cardiac function. LMW FGF2-mediated cardioprotection is facilitated by activation of multiple kinases, including PKCalpha, PKCepsilon, and ERK, and inhibition of p38 and JNK.
Objective
Yet, the substrates of those kinases associated with LMW FGF2-induced cardioprotection against myocardial dysfunction remain to be elucidated.
Methods and results
To identify substrates in LMW FGF2 improvement of post-ischemic cardiac function, mouse hearts expressing only LMW FGF2 were subjected to ischemia-reperfusion (I/R) injury and analyzed by a mass spectrometry (MS)-based quantitative phosphoproteomic strategy. MS analysis identified 50 phosphorylation sites from 7 sarcoendoplasmic reticulum (SR) proteins that were significantly altered in I/R-treated hearts only expressing LMW FGF2 compared to those hearts lacking FGF2. One of those phosphorylated SR proteins identified was phospholamban (PLB), which exhibited rapid, increased phosphorylation at Threonine-17 (Thr17) after I/R in hearts expressing only LMW FGF2; this was further validated using Selected Reaction Monitoring-based MS workflow. To demonstrate a mechanistic role of phospho-Thr17 PLB in LMW FGF2-mediated cardioprotection, hearts only expressing LMW FGF2 and those expressing only LMW FGF2 with a mutant PLB lacking phosphorylatable Thr17 (Thr17Ala PLB) were subjected to I/R. Hearts only expressing LMW FGF2 showed significantly improved recovery of cardiac function following I/R (p < 0.05), and this functional improvement was significantly abrogated in hearts expressing LMW FGF2 and Thr17Ala PLB (p < 0.05).
Conclusion
The findings indicate that LMW FGF2 modulates intracellular calcium handling/cycling via regulatory changes in SR proteins essential for recovery from I/R injury, and thereby protects the heart from post-ischemic cardiac dysfunction.
中文翻译:

磷酸化蛋白质组学分析确定了受磷蛋白的 phospho-Threonine-17 位点,该位点在成纤维细胞生长因子 2 的低分子量异构体诱导的缺血后心脏功能障碍保护中很重要。
基本原理
在其众多生物学作用中,成纤维细胞生长因子 2 (FGF2) 可保护心脏免受与缺血发作相关的功能障碍和损伤。我们的实验室证明其对心肌功能障碍的保护作用是通过 FGF2 的低分子量 (LMW) 异构体实现的,而高分子量 (HMW) 异构体与缺血后心脏功能恢复的恶化有关。LMW FGF2 介导的心脏保护作用通过激活多种激酶(包括 PKCalpha、PKCepsilon 和 ERK)以及抑制 p38 和 JNK 来促进。
客观的
然而,与 LMW FGF2 诱导的对心肌功能障碍的心脏保护作用相关的那些激酶的底物仍有待阐明。
方法和结果
为了确定 LMW FGF2 改善缺血后心脏功能的底物,对仅表达 LMW FGF2 的小鼠心脏进行缺血再灌注 (I/R) 损伤,并通过基于质谱 (MS) 的定量磷酸蛋白质组学策略进行分析。MS 分析从 7 种肌内质网 (SR) 蛋白中鉴定出 50 个磷酸化位点,与缺乏 FGF2 的心脏相比,这些蛋白在仅表达 LMW FGF2 的 I/R 处理的心脏中发生了显着改变。已鉴定的磷酸化 SR 蛋白之一是受磷蛋白 (PLB),它在仅表达 LMW FGF2 的心脏中 I/R 后在苏氨酸 17 (Thr17) 处表现出快速、增加的磷酸化。使用基于选择反应监测的 MS 工作流程进一步验证了这一点。为了证明磷酸化 Thr17 PLB 在 LMW FGF2 介导的心脏保护中的机制作用,仅表达LMW FGF2的心脏和仅表达LMW FGF2且具有缺乏可磷酸化Thr17(Thr17Ala PLB)的突变PLB的心脏进行I / R。仅表达 LMW FGF2 的心脏在 I/R 后显示出显着改善的心脏功能恢复(p < 0.05),并且这种功能改善在表达 LMW FGF2 和 Thr17Ala PLB 的心脏中明显消失 ( p < 0.05)。
结论
研究结果表明,LMW FGF2 通过调节 I/R 损伤恢复所必需的 SR 蛋白的调节变化来调节细胞内钙处理/循环,从而保护心脏免受缺血后心脏功能障碍的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号