当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
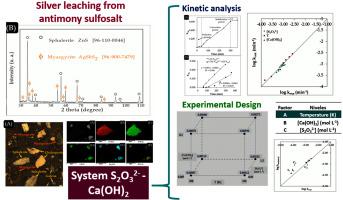
Silver leaching from miargyrite (AgSbS2) sulfosalt in the system S2O32−-ca(OH)2: Kinetic analysis and experimental design approach
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105456 A.M. Teja-Ruiz , I.A. Reyes-Domínguez , O.A. Acevedo-Sandoval , E.G. Palacios-Beas , M.U. Flores-Guerrero , M. Pérez-Labra , J.C. Juárez-Tapia
Hydrometallurgy ( IF 4.7 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105456 A.M. Teja-Ruiz , I.A. Reyes-Domínguez , O.A. Acevedo-Sandoval , E.G. Palacios-Beas , M.U. Flores-Guerrero , M. Pérez-Labra , J.C. Juárez-Tapia
|
Abstract This investigation reports the silver dissolution kinetics from a ore-type sulfosalt identified as miargyrite, which prevails in the chemical concentration process of polymetallic concentrates, in the S2O32−-Ca(OH)2 system as well as a statistical study of the design of experiments concerning the effect of temperature (T), calcium hydroxide concentration ([Ca(OH)2]), thiosulfate concentration ([S2O32−]), particle size (d0) and stirring speed (RPM). The results show that temperature is the variable with the most significant effect on the dissolution rate of silver and obtains an activation energy (Ea) of 57.19 kJ mol−1, followed by particle size and hydroxide concentration. Furthermore, it was observed that stirring speed does not affect the dissolution rates in basic medium. These results are consistent and demonstrate that the dissolution reaction is controlled by the chemical reaction. The study of the factorial design was relevant to elucidate the behavior of the variables with the greatest impact in the experimental kinetic study. Finally, kinetic and statistical models were proposed to describe the silver dissolution process in an alkaline medium. The highest silver dissolution was achieved using the following conditions V = 0.5 L, mineral = 40 g L−1, PO2 = 1 atm, t = 360 min, d0 = −105 + 74 μm, [S2O32−] = 0.5 mol L−1, [Ca(OH)2] = 0.05 mol L−1, T = 338 K and RPM = 670 min−1.
中文翻译:

在 S2O32−-ca(OH)2 系统中从水银矿 (AgSbS2) 磺盐中浸出银:动力学分析和实验设计方法
摘要 本研究报告了在 S2O32--Ca(OH)2 系统中多金属精矿的化学浓缩过程中普遍存在的一种被鉴定为水银矿的矿石型硫盐的银溶解动力学,以及对关于温度 (T)、氢氧化钙浓度 ([Ca(OH)2])、硫代硫酸盐浓度 ([S2O32−])、粒径 (d0) 和搅拌速度 (RPM) 影响的实验。结果表明,温度是对银的溶解速率影响最显着的变量,获得的活化能 (Ea) 为 57.19 kJ mol-1,其次是粒径和氢氧化物浓度。此外,观察到搅拌速度不影响在碱性介质中的溶解速率。这些结果是一致的,表明溶解反应是由化学反应控制的。因子设计的研究与阐明在实验动力学研究中影响最大的变量的行为有关。最后,提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。
更新日期:2020-12-01
中文翻译:

在 S2O32−-ca(OH)2 系统中从水银矿 (AgSbS2) 磺盐中浸出银:动力学分析和实验设计方法
摘要 本研究报告了在 S2O32--Ca(OH)2 系统中多金属精矿的化学浓缩过程中普遍存在的一种被鉴定为水银矿的矿石型硫盐的银溶解动力学,以及对关于温度 (T)、氢氧化钙浓度 ([Ca(OH)2])、硫代硫酸盐浓度 ([S2O32−])、粒径 (d0) 和搅拌速度 (RPM) 影响的实验。结果表明,温度是对银的溶解速率影响最显着的变量,获得的活化能 (Ea) 为 57.19 kJ mol-1,其次是粒径和氢氧化物浓度。此外,观察到搅拌速度不影响在碱性介质中的溶解速率。这些结果是一致的,表明溶解反应是由化学反应控制的。因子设计的研究与阐明在实验动力学研究中影响最大的变量的行为有关。最后,提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。提出了动力学和统计模型来描述碱性介质中的银溶解过程。使用以下条件实现最高的银溶解 V = 0.5 L,矿物 = 40 g L−1,PO2 = 1 atm,t = 360 min,d0 = -105 + 74 μm,[S2O32−] = 0.5 mol L− 1,[Ca(OH)2] = 0.05 mol L−1,T = 338 K 和 RPM = 670 min−1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号