European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-08-23 , DOI: 10.1016/j.ejmech.2020.112771 David L Cramer 1 , Bo Cheng 1 , Jianhua Tian 1 , John H Clements 1 , Rachel M Wypych 1 , Stephen F Martin 1
|
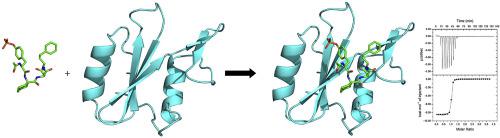
Understanding how making structural changes in small molecules affects their binding affinities for targeted proteins is central to improving strategies for rational drug design. To assess the effects of varying the nature of nonpolar groups upon binding entropies and enthalpies, we designed and prepared a set of Grb2-SH2 domain ligands, Ac–pTyr–Ac6c–Asn–(CH2)n–R, in which the size and electrostatic nature of R groups at the pTyr+3 site were varied. The complexes of these ligands with the Grb2-SH2 domain were evaluated in a series of studies in which the binding thermodynamics were determined using isothermal titration calorimetry, and binding interactions were examined in crystallographic studies of two different complexes. Notably, adding nonpolar groups to the pTyr+3 site leads to higher binding affinities, but the magnitude and energetic origins of these effects vary with the nature of the R substituent. For example, enhancements to binding affinities using aliphatic R groups are driven by more favorable changes in binding entropies, whereas aryl R groups improve binding free energies through a combination of more favorable changes in binding enthalpies and entropies. However, enthalpy/entropy compensation plays a significant role in these associations and mitigates against any significant variation in binding free energies, which vary by only 0.8 kcal•mol−1, with changes in the electrostatic nature and size of the R group. Crystallographic studies show that differences in ΔG° or ΔH° correlate with buried nonpolar surface area, but they do not correlate with the total number of polar or van der Waals contacts. The relative number of ordered water molecules and relative order in the side chains at pTyr+3 correlate with differences in –TΔS°. Overall, these studies show that burial of nonpolar surface can lead to enhanced binding affinities arising from dominating entropy- or enthalpy-driven hydrophobic effects, depending upon the electrostatic nature of the apolar R group.
中文翻译:

蛋白质-配体相互作用中不同非极性表面的一些热力学效应。
了解小分子的结构变化如何影响它们对靶蛋白的结合亲和力,对于改进合理的药物设计策略至关重要。为了评估改变非极性基团的性质对结合熵和焓的影响,我们设计并制备了一组 Grb2-SH2 结构域配体 Ac–pTyr–Ac 6 c–Asn–(CH 2 ) n – R,其中R的大小和静电性质pTyr+3 位点的组是不同的。这些配体与 Grb2-SH2 结构域的复合物在一系列研究中进行了评估,其中使用等温滴定量热法确定了结合热力学,并在两种不同复合物的晶体学研究中检查了结合相互作用。值得注意的是,将非极性基团添加到 pTyr+3 位点会导致更高的结合亲和力,但这些影响的大小和能量来源随R取代基的性质而变化。例如,使用脂肪族R基团增强结合亲和力是由结合熵的更有利变化驱动的,而芳基R组通过结合焓和熵的更有利变化的组合来提高结合自由能。然而,焓/熵补偿在这些关联中起着重要作用,并减轻了结合自由能的任何显着变化,结合自由能仅变化 0.8 kcal•mol -1,随R基团的静电性质和大小的变化。晶体学研究表明,Δ G ° 或 Δ H ° 的差异与埋藏的非极性表面积相关,但它们与极性或范德华接触的总数无关。pTyr+3 处有序水分子的相对数量和侧链中的相对顺序与-TΔ S 的差异相关°。总的来说,这些研究表明,根据非极性R基团的静电性质,非极性表面的掩埋会导致由主导的熵或焓驱动的疏水效应引起的增强的结合亲和力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号