当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric total synthesis of dihydroisocoumarins: 6-methoxymellein, kigelin and fusarentin 6, 7 dimethyl ether by employing proline catalysed asymmetric α-aminoxylation
Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-23 , DOI: 10.1016/j.tet.2020.131524 Sachin B. Markad , Baliram B. Mane , Suresh B. Waghmode
中文翻译:

二氢异香豆素的不对称全合成:脯氨酸催化的不对称α-氨基木糖基化反应生成6-甲氧基水elle素,kigelin和fusrentin 6、7二甲醚
更新日期:2020-08-23
Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-23 , DOI: 10.1016/j.tet.2020.131524 Sachin B. Markad , Baliram B. Mane , Suresh B. Waghmode
|
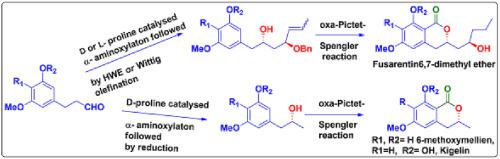
A concise asymmetric total synthesis of dihydroisocoumarins such as 6-methoxymellein, kigelin and fusarentin 6,7 dimethyl ether in high enantiopurity have been achieved from non-chiral aldehydes by employing proline catalysed asymmetric α-aminoxylation reaction. The required stereochemistry of hydroxyl group have been generated by alternating L or D proline as a organocatalyst in α-aminoxylation step and lactone ring is assembled by oxa-Pictet-Spengler cyclisation reaction as the key steps.
中文翻译:

二氢异香豆素的不对称全合成:脯氨酸催化的不对称α-氨基木糖基化反应生成6-甲氧基水elle素,kigelin和fusrentin 6、7二甲醚
通过使用脯氨酸催化的不对称α-氨基木糖基化反应,可以从非手性醛类化合物中以高对映体纯度进行简明的不对称全合成二氢异香豆素(如6-甲氧基水杨素,kigelin和fusentin 6,7二甲醚)。所需的羟基立体化学是通过在L-氨氧基化步骤中交替使用L或D脯氨酸作为有机催化剂而生成的,内酯环是通过oxa-Pictet-Spengler环化反应组装的关键步骤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号