Biomaterials ( IF 14.0 ) Pub Date : 2020-08-22 , DOI: 10.1016/j.biomaterials.2020.120335 Inge Mannaerts 1 , Nathalie Eysackers 1 , Elise Anne van Os 1 , Stefaan Verhulst 1 , Tiffany Roosens 1 , Ayla Smout 1 , Andreas Hierlemann 2 , Olivier Frey 3 , Sofia Batista Leite 1 , Leo A van Grunsven 1
|
A major obstacle in the development of efficient therapies for progressive liver fibrosis is the lack of representative in vitro models of liver fibrosis to aid in understanding the mechanisms of the disease and to promote the development of pharmaceuticals.
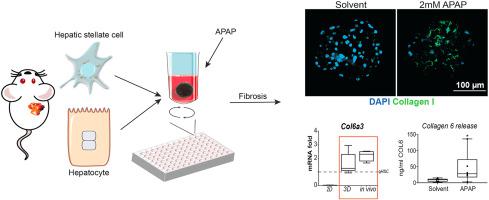
Our aim was to develop a relevant in vitro mouse liver fibrosis model, based on the central hypothesis that liver fibrosis in vitro cannot be studied using only hepatic stellate cells (HSCs)–the main producer of scar tissue during fibrosis–, but requires cultures in which at least hepatocytes are integrated.
We established robust methods to generate co-culture spheroids from freshly isolated mouse hepatocytes and HSCs. Characteristics and functionality of these spheroids were analyzed by qPCR of cell-type specific markers, CYP induction and immunohistochemistry. Compound toxicity was determined by ATP-assays.
Hepatocytes and HSCs maintained their cell-type specific marker expression over a 15-day culture period without major hepatocyte dedifferentiation or HSC activation. Exposure of spheroids to TGFβ can directly activate HSCs, while acetaminophen exposure mounts a hepatocyte damage dependent activation of HSCs. Pharmaceuticals with known anti-fibrotic properties, such as Valproic acid and Verteporfin, reduce HSC activation in response to hepatocyte damage in these cultures. A comparison between the fibrotic response of the spheroid co-cultures and in vivo activated HSCs showed that these 3D co-cultures are more representative than the commonly used 2D HSC monocultures. Finally, we showed that the 3D cultures can be integrated in microfluidic chips.
We conclude that our hepatocyte-stellate cell-spheroid cultures are a robust in vitro model of liver fibrosis. This model could be used to further unravel the mechanism of HSC activation and facilitate the discovery of, or testing for novel anti-fibrotic compounds, as these spheroids better reproduce HSC in vivo activation compared to the more traditional 2D mono-culture models.
中文翻译:

原发性肝球体的纤维化反应概括了体内肝星状细胞的活化。
发展有效的进行性肝纤维化疗法的主要障碍是缺乏代表性的肝纤维化体外模型,以帮助理解疾病的机制并促进药物的开发。
我们的目标是建立一个相关的体外小鼠肝纤维化模型,其主要假设是,不能仅使用肝星状细胞(HSC)(在纤维化过程中瘢痕组织的主要产生者)研究体外肝纤维化,而是需要进行体外培养。至少整合了肝细胞。
我们建立了可靠的方法,可以从新鲜分离的小鼠肝细胞和HSC产生共培养的球体。通过细胞类型特异性标记物的qPCR,CYP诱导和免疫组化分析这些球体的特征和功能。通过ATP测定来确定化合物的毒性。
肝细胞和HSC在15天的培养期内保持其细胞类型特异性标志物的表达,而没有主要的肝细胞去分化或HSC活化。将球状体暴露于TGFβ可以直接激活HSC,而对乙酰氨基酚暴露则增加了肝细胞依赖的HSC激活。具有已知抗纤维化特性的药物(如丙戊酸和维替泊芬)在这些培养物中可响应肝细胞损伤而降低HSC活化。球状共培养物与体内活化的HSC的纤维化反应之间的比较表明,这些3D共培养物比常用的2D HSC单培养物更具代表性。最后,我们证明了3D培养物可以整合到微流控芯片中。
我们得出的结论是,我们的肝细胞星状细胞球状培养物是肝纤维化的强大体外模型。此模型可用于进一步阐明HSC激活的机制,并促进发现或测试新型抗纤维化化合物,因为与传统的2D单培养模型相比,这些球体可更好地重现HSC体内激活。


























 京公网安备 11010802027423号
京公网安备 11010802027423号