当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reductive Electrochemical Activation of Hydrogen Peroxide as an Advanced Oxidation Process for Treatment of Reverse Osmosis Permeate during Potable Reuse.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.est.0c02144 Cindy Weng,Yi-Hsueh Chuang,Bradley Davey,William A Mitch
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.est.0c02144 Cindy Weng,Yi-Hsueh Chuang,Bradley Davey,William A Mitch
|
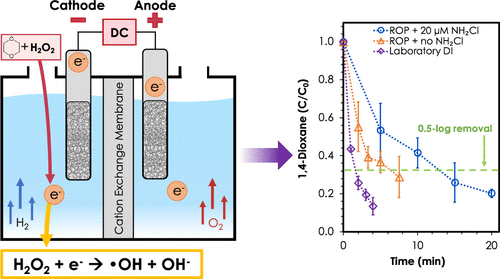
The UV/hydrogen peroxide (H2O2) advanced oxidation process (AOP) frequently employed to generate hydroxyl radical (•OH) to treat reverse osmosis permeate (ROP) in potable reuse treatment trains is inefficient, using only 10% of the H2O2. This study evaluated ·OH generation by electron transfer from a low-cost stainless steel cathode. In deionized water, the electrochemical system achieved 0.5 log removal of 1,4-dioxane, a benchmark for AOP validation for potable reuse, within 4 min using only 1.25 mg/L H2O2. Hydrogen peroxide and 1,4-dioxane degradations were maximized near −0.18 and + 0.02 V versus standard hydrogen electrode, respectively. Degradations of positively and negatively charged compounds were comparable to neutral 1,4-dioxane, indicating that degradation occurs by ·OH generation from neutral H2O2 and that electrostatic repulsion of contaminants from the electrode is not problematic. For ROP without chloramines, 0.5 log 1,4-dioxane removal was achieved in 6.7 min with 7 mM salts for ionic strength and 2.5 mg/L H2O2. For ROP with 1.4 mg/L as Cl2 chloramines, 0.5 log 1,4-dioxane removal was achieved in 13.2 min with 7 mM salts and 4.5 mg/L total H2O2 dosed in three separate injections in 5 min intervals. Initial estimates based on lab-scale electrochemical AOP treatment indicated that, except for the cost of salts, the electrochemical AOP featured lower reagent costs than the UV/H2O2 AOP but higher electricity costs that could be reduced by optimization of the electrochemical design.
中文翻译:

过氧化氢的还原性电化学活化作为高级氧化工艺,用于在饮用水回用过程中处理反渗透渗透液。
经常使用的紫外线/过氧化氢(H 2 O 2)高级氧化工艺(AOP)产生羟基自由基(• OH)来处理便携式重复使用处理系统中的反渗透渗透液(ROP)效率低下,仅使用10%的H 2 O 2。这项研究评估了·低成本不锈钢阴极通过电子转移产生的OH。在去离子水中,电化学系统仅使用1.25 mg / LH 2 O 2即可在4分钟内去除0.5 log的1,4-二恶烷,这是AOP验证可重复使用的基准。与标准氢电极相比,分别在-0.18和+ 0.02 V附近使过氧化氢和1,4-二恶烷的降解最大化。正电和带负电荷的化合物的降解比得上中性1,4-二恶烷,指示由发生的降解· OH从中立ħ代2 ö 2和从电极污染物的那静电排斥是没有问题的。对于不含氯胺的ROP,在6.7分钟内用7 mM的离子强度盐和2.5 mg / LH 2 O 2盐去除0.5 log 1,4-二恶烷。对于含1.4 mg / L的Cl 2氯胺的ROP,在13.2分钟内用7 mM的盐和4.5 mg / L的总H 2 O去除了0.5 log 1,4-二恶烷2剂,每5分钟间隔3次注射。基于实验室规模的电化学AOP处理的初步估计表明,除了盐的成本外,电化学AOP的试剂成本比UV / H 2 O 2 AOP低,但可以通过优化电化学设计来降低电费。
更新日期:2020-10-06
中文翻译:

过氧化氢的还原性电化学活化作为高级氧化工艺,用于在饮用水回用过程中处理反渗透渗透液。
经常使用的紫外线/过氧化氢(H 2 O 2)高级氧化工艺(AOP)产生羟基自由基(• OH)来处理便携式重复使用处理系统中的反渗透渗透液(ROP)效率低下,仅使用10%的H 2 O 2。这项研究评估了·低成本不锈钢阴极通过电子转移产生的OH。在去离子水中,电化学系统仅使用1.25 mg / LH 2 O 2即可在4分钟内去除0.5 log的1,4-二恶烷,这是AOP验证可重复使用的基准。与标准氢电极相比,分别在-0.18和+ 0.02 V附近使过氧化氢和1,4-二恶烷的降解最大化。正电和带负电荷的化合物的降解比得上中性1,4-二恶烷,指示由发生的降解· OH从中立ħ代2 ö 2和从电极污染物的那静电排斥是没有问题的。对于不含氯胺的ROP,在6.7分钟内用7 mM的离子强度盐和2.5 mg / LH 2 O 2盐去除0.5 log 1,4-二恶烷。对于含1.4 mg / L的Cl 2氯胺的ROP,在13.2分钟内用7 mM的盐和4.5 mg / L的总H 2 O去除了0.5 log 1,4-二恶烷2剂,每5分钟间隔3次注射。基于实验室规模的电化学AOP处理的初步估计表明,除了盐的成本外,电化学AOP的试剂成本比UV / H 2 O 2 AOP低,但可以通过优化电化学设计来降低电费。

























 京公网安备 11010802027423号
京公网安备 11010802027423号