当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dimeric and esterified sesquiterpenes from the liverwort Chiastocaulon caledonicum
Phytochemistry ( IF 3.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.phytochem.2020.112495 Benjamin Métoyer 1 , Annecie Benatrehina 2 , L Harinantenaina Rakotondraibe 2 , Louis Thouvenot 3 , Yoshinori Asakawa 4 , Mohammed Nour 5 , Phila Raharivelomanana 6
Phytochemistry ( IF 3.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.phytochem.2020.112495 Benjamin Métoyer 1 , Annecie Benatrehina 2 , L Harinantenaina Rakotondraibe 2 , Louis Thouvenot 3 , Yoshinori Asakawa 4 , Mohammed Nour 5 , Phila Raharivelomanana 6
Affiliation
|
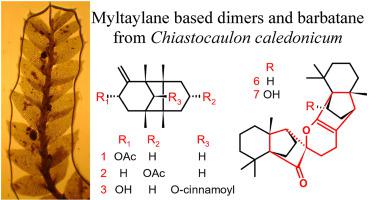
This is the first chemical investigation of Chiastocaulon caledonicum, an endemic liverwort from New Caledonia. We herein present the isolation of thirteen compounds including seven undescribed sesquiterpenoids, namely four barbatane- and three myltaylane-type sesquiterpenes. The structures of these compounds were elucidated based on the interpretation of their chemical and spectroscopic/spectrometric data. Chiastocaulins A and B are the first examples of dimers based on two myltaylane units. The chemotaxonomic importance and the biosynthesis of the chiastocaulin structure are discussed. Terpenoid dimers formed via a Diels-Alder cyclization are thought to be specific to the Plagiochilaceae family.
中文翻译:

来自地草 Chiastocaulon caledonicum 的二聚体和酯化倍半萜烯
这是对来自新喀里多尼亚的地方性苔草 Chiastocaulon caledonicum 的首次化学研究。我们在此介绍了 13 种化合物的分离,包括 7 种未描述的倍半萜类化合物,即四种 barbatane 和三种 myltaylane 型倍半萜。基于对它们的化学和光谱/光谱数据的解释,阐明了这些化合物的结构。Chiastocaulins A 和 B 是基于两个 mytaylane 单元的二聚体的第一个例子。讨论了化学分类学的重要性和 chiastocaulin 结构的生物合成。通过 Diels-Alder 环化形成的萜类二聚体被认为是 Plagiochilaceae 家族特有的。
更新日期:2020-11-01
中文翻译:

来自地草 Chiastocaulon caledonicum 的二聚体和酯化倍半萜烯
这是对来自新喀里多尼亚的地方性苔草 Chiastocaulon caledonicum 的首次化学研究。我们在此介绍了 13 种化合物的分离,包括 7 种未描述的倍半萜类化合物,即四种 barbatane 和三种 myltaylane 型倍半萜。基于对它们的化学和光谱/光谱数据的解释,阐明了这些化合物的结构。Chiastocaulins A 和 B 是基于两个 mytaylane 单元的二聚体的第一个例子。讨论了化学分类学的重要性和 chiastocaulin 结构的生物合成。通过 Diels-Alder 环化形成的萜类二聚体被认为是 Plagiochilaceae 家族特有的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号