当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
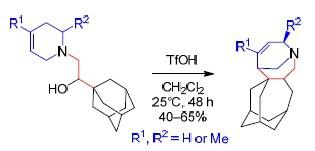
Transformations of 1-[2-(Adamantan-1-Yl)-2-Hydroxyethyl]-1,2,3,6-Tetrahydropyridines by the Action of Trifluoromethanesulfonic Acid
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-08-22 , DOI: 10.1007/s10593-020-02747-9 Vera А. Shadrikova , Evgeny V. Golovin , Victor B. Rybakov , Yuri N. Klimochkin

中文翻译:

三氟甲磺酸作用下的1- [2-(金刚烷-1-基)-2-羟基乙基] -1,2,3,6-四氢吡啶的转化

Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-08-22 , DOI: 10.1007/s10593-020-02747-9 Vera А. Shadrikova , Evgeny V. Golovin , Victor B. Rybakov , Yuri N. Klimochkin
|

中文翻译:

三氟甲磺酸作用下的1- [2-(金刚烷-1-基)-2-羟基乙基] -1,2,3,6-四氢吡啶的转化



























 京公网安备 11010802027423号
京公网安备 11010802027423号