当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
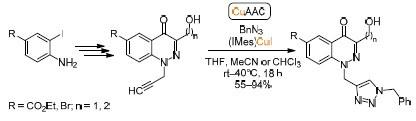
N -Propargylation and Copper(I)-Catalyzed Azide-Alkyne Cycloaddition as a Convenient Strategy for Directed Post-Synthetic Modification of 4-Oxo-1,4-Dihydrocinnoline Derivatives
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-08-22 , DOI: 10.1007/s10593-020-02750-0 Vladimir N. Mikhaylov , Artem O. Pavlov , Yaroslav V. Ogorodnov , Dar’ya V. Spiridonova , Viktor N. Sorokoumov , Irina A. Balova

中文翻译:

N-炔丙基化和铜(I)催化的叠氮化物-炔烃环加成反应是4-Oxo-1,4-Dihydrocinnoline衍生物的直接合成后修饰的简便策略

Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-08-22 , DOI: 10.1007/s10593-020-02750-0 Vladimir N. Mikhaylov , Artem O. Pavlov , Yaroslav V. Ogorodnov , Dar’ya V. Spiridonova , Viktor N. Sorokoumov , Irina A. Balova
|

中文翻译:

N-炔丙基化和铜(I)催化的叠氮化物-炔烃环加成反应是4-Oxo-1,4-Dihydrocinnoline衍生物的直接合成后修饰的简便策略



























 京公网安备 11010802027423号
京公网安备 11010802027423号