当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photonic hyperthermal and sonodynamic nanotherapy targeting oral squamous cell carcinoma.
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0tb01089h Jiaxin Zuo 1 , Minfeng Huo 2 , Liying Wang 2 , Jia Li 1 , Yu Chen 3 , Ping Xiong 1
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0tb01089h Jiaxin Zuo 1 , Minfeng Huo 2 , Liying Wang 2 , Jia Li 1 , Yu Chen 3 , Ping Xiong 1
Affiliation
|
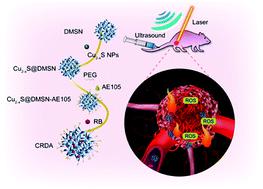
Nanomedicine that enables multiple synergetic treatments provides effective non-invasive treatment modalities for cancer therapy. Yet treatments for oral squamous cell carcinoma (OSCC) are rarely reported. Here, we designed OSCC-targeting multi-functional nanomedicines to overcome the therapeutic obstacles during OSCC treatments, including ineffective chemotherapy, and the traumatic surgery and radiotherapy. The urokinase plasminogen activator receptor (uPAR)-targeting ligand AE105 decorated dendritic mesoporous silica nanoparticles (DMSN) encapsulating photonic active ultrasmall Cu2−xS NPs and sonosensitizer Rose Bengal (RB) have been rationally designed and constructed (designated as Cu2−xS-RB@DMSN-AE105, abbreviated as CRDA). These CRDAs initially target the uPAR, which is overexpressed in the OSCC cell membrane, to increase the localized accumulation of CRDAs at tumor sites. Under the irradiation of both near-infrared laser and ultrasound, the in situ photonic-hyperthermal and sonodynamic effects are respectively enabled to induce the cell death of OSCC. Upon both in vitro/in vivo challenges, tumor cells/xenografts have been efficiently eradicated, achieving the targeting and synergetic treatment modality against the OSCC with satisfactory biocompatibility.
中文翻译:

针对口腔鳞状细胞癌的光子高温和声动力学纳米疗法。
能够进行多种协同治疗的纳米药物为癌症治疗提供了有效的非侵入性治疗方式。然而,很少报道口腔鳞状细胞癌(OSCC)的治疗方法。在这里,我们设计了靶向OSCC的多功能纳米药物,以克服OSCC治疗期间的治疗障碍,包括无效的化学疗法以及创伤性手术和放疗。针对尿激酶纤溶酶原激活剂受体(uPAR)的配体AE105装饰的树状介孔二氧化硅纳米颗粒(DMSN)封装了光子活性超小Cu 2- x S NP和声敏剂Rose Bengal(RB)(已设计为Cu 2- xS-RB @ DMSN-AE105,缩写为CRDA)。这些CRDA最初靶向在OSCC细胞膜中过表达的uPAR,以增加CRDA在肿瘤部位的局部积累。在近红外激光和超声的照射下,原位光子超热效应和声动力学效应分别能够诱导OSCC细胞死亡。一旦二者在体外/体内挑战,肿瘤细胞/异种移植物已被有效地消除,从而实现针对具有满意的生物相容性的OSCC靶向和协同的治疗模式。
更新日期:2020-10-14
中文翻译:

针对口腔鳞状细胞癌的光子高温和声动力学纳米疗法。
能够进行多种协同治疗的纳米药物为癌症治疗提供了有效的非侵入性治疗方式。然而,很少报道口腔鳞状细胞癌(OSCC)的治疗方法。在这里,我们设计了靶向OSCC的多功能纳米药物,以克服OSCC治疗期间的治疗障碍,包括无效的化学疗法以及创伤性手术和放疗。针对尿激酶纤溶酶原激活剂受体(uPAR)的配体AE105装饰的树状介孔二氧化硅纳米颗粒(DMSN)封装了光子活性超小Cu 2- x S NP和声敏剂Rose Bengal(RB)(已设计为Cu 2- xS-RB @ DMSN-AE105,缩写为CRDA)。这些CRDA最初靶向在OSCC细胞膜中过表达的uPAR,以增加CRDA在肿瘤部位的局部积累。在近红外激光和超声的照射下,原位光子超热效应和声动力学效应分别能够诱导OSCC细胞死亡。一旦二者在体外/体内挑战,肿瘤细胞/异种移植物已被有效地消除,从而实现针对具有满意的生物相容性的OSCC靶向和协同的治疗模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号