当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrodeoxygenation of vegetable oils over biochar supported bimetallic carbides for producing renewable diesel under mild conditions
Green Chemistry ( IF 9.8 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0gc00680g Chi-Cong Tran 1, 2, 3, 4 , Dahi Akmach 5, 6, 7, 8, 9 , Serge Kaliaguine 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2020-08-21 , DOI: 10.1039/d0gc00680g Chi-Cong Tran 1, 2, 3, 4 , Dahi Akmach 5, 6, 7, 8, 9 , Serge Kaliaguine 1, 2, 3, 4
Affiliation
|
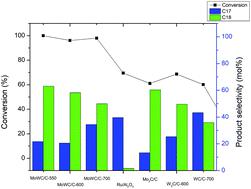
Bimetallic Mo–W carbides supported on biochar were synthesized and used in the catalytic hydrotreatment of canola oil at 250 °C to produce diesel-range hydrocarbons. The effects of carburization temperature and metal content on the nature of active sites were investigated by using X-ray diffraction (XRD), N2 physisorption, X-ray photoelectron spectroscopy (XPS), and CO and H2 chemisorption. Varying temperature over the range of 550–700 °C did not have any effect on the formation of the Mo2C phase in the bimetallic carbide. As for the tungsten component in the mixed carbide, formation of the WC phase at a high temperature of 700 °C was dominant and increased the density of hydrogen activating sites, whereas at lower temperatures (≤600 °C), W2C and metallic W phases were formed and showed more CO adsorption sites. Increasing metal loading enhanced the particle size resulting in a lower density of catalytically active sites. The addition of W into the molybdenum carbide system strongly increased the catalytic performance with >95% conversion and >76% hydrocarbon yield over all mixed metal carbides at a mild temperature of 250 °C. These values were both higher than those obtained using Mo2C/C and Ru/Al2O3 (48 and 35% hydrocarbon yield, respectively) under identical conditions. All carbide catalysts favor hydrodeoxygenation (HDO) products over decarboxylation/decarbonylation (DCO) products; however, W addition into mixed metal carbides increases the DCO selectivity in comparison with Mo2C/C due to higher ratios of H2/CO adsorption sites. The bimetallic carbides still retained a high catalyst activity after regeneration.
中文翻译:

在生物炭负载的双金属碳化物上对植物油进行加氢脱氧,以在温和条件下生产可再生柴油
合成了负载在生物炭上的双金属Mo-W碳化物,并将其用于菜籽油在250°C的催化加氢处理中,以生产柴油范围的碳氢化合物。通过X射线衍射(XRD),N 2物理吸附,X射线光电子能谱(XPS)以及CO和H 2的化学吸附研究了渗碳温度和金属含量对活性位点性质的影响。在550–700°C范围内变化的温度对双金属碳化物中Mo 2 C相的形成没有任何影响。至于混合碳化物中的钨成分,在700°C的高温下WC相的形成占主导地位,并增加了氢活化位点的密度,而在较低的温度(≤600°C)下,W 2C和金属W相形成并显示出更多的CO吸附位点。金属负载的增加增加了粒径,导致较低的催化活性位点密度。在250°C的温和温度下,将W添加到碳化钼体系中,与所有混合金属碳化物相比,转化率> 95%,烃收率> 76%,大大提高了催化性能。这些值均高于在相同条件下使用Mo 2 C / C和Ru / Al 2 O 3获得的值(分别为48和35%的烃产率)。所有的碳化物催化剂都喜欢加氢脱氧(HDO)产物,而不是脱羧/脱羰基(DCO)产物。然而,与Mo 2相比,向混合金属碳化物中添加W会增加DCO选择性C / C是由于H 2 / CO吸附位点的比例较高。再生后,双金属碳化物仍保持高催化剂活性。
更新日期:2020-10-05
中文翻译:

在生物炭负载的双金属碳化物上对植物油进行加氢脱氧,以在温和条件下生产可再生柴油
合成了负载在生物炭上的双金属Mo-W碳化物,并将其用于菜籽油在250°C的催化加氢处理中,以生产柴油范围的碳氢化合物。通过X射线衍射(XRD),N 2物理吸附,X射线光电子能谱(XPS)以及CO和H 2的化学吸附研究了渗碳温度和金属含量对活性位点性质的影响。在550–700°C范围内变化的温度对双金属碳化物中Mo 2 C相的形成没有任何影响。至于混合碳化物中的钨成分,在700°C的高温下WC相的形成占主导地位,并增加了氢活化位点的密度,而在较低的温度(≤600°C)下,W 2C和金属W相形成并显示出更多的CO吸附位点。金属负载的增加增加了粒径,导致较低的催化活性位点密度。在250°C的温和温度下,将W添加到碳化钼体系中,与所有混合金属碳化物相比,转化率> 95%,烃收率> 76%,大大提高了催化性能。这些值均高于在相同条件下使用Mo 2 C / C和Ru / Al 2 O 3获得的值(分别为48和35%的烃产率)。所有的碳化物催化剂都喜欢加氢脱氧(HDO)产物,而不是脱羧/脱羰基(DCO)产物。然而,与Mo 2相比,向混合金属碳化物中添加W会增加DCO选择性C / C是由于H 2 / CO吸附位点的比例较高。再生后,双金属碳化物仍保持高催化剂活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号