当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Practical, Biocatalytic Synthesis of tert-Butyl (R)-3-Hydroxyl-5-hexenoate: A Key Intermediate to the Statin Side Chain
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.oprd.0c00320 Chen Hu 1, 2 , Minjie Liu 1, 2 , Xiaoping Yue 3 , Zedu Huang 1, 2 , Fener Chen 1, 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-08-21 , DOI: 10.1021/acs.oprd.0c00320 Chen Hu 1, 2 , Minjie Liu 1, 2 , Xiaoping Yue 3 , Zedu Huang 1, 2 , Fener Chen 1, 2
Affiliation
|
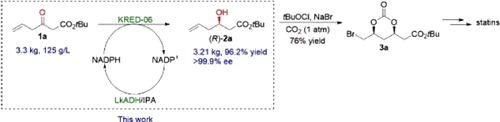
The HMG-CoA reductase inhibitors, statins, are one of the most effective and bestselling cholesterol-lowering drugs. The use of statins has greatly extended people’s lives and improved the quality of their life. Development of a more efficient, stereoselective, and sustainable synthesis of statins is continuingly of utmost importance. In the present study, through screening of ketoreductases (KREDs) and reaction optimization, we have successfully performed a highly stereoselective reduction of ketoester 1a catalyzed by KRED-06 at a pilot-plant scale without the addition of exogenous NADP+, generating 3.21 kg of enantiomerically pure tert-butyl (R)-3-hydroxyl-5-hexenoate ((R)-2a) (96.2% yield, >99.9% enantiomeric excess (ee)). This newly developed biocatalytic process alleviates the cryogenic conditions (−40 °C) employed in our first-generation synthesis of (R)-2a using NaBH4 and (l)-tartaric acid. Coupled with our previously established synthesis of bromocarbonate 3a via a one-pot diastereoselective carboxylation/bromocyclization of (R)-2a, we have developed an innovative, practical synthesis route to statin side chain, possessing great potential to be implemented into industrial production of statins.
中文翻译:

实用的生物催化合成叔丁基(R)-3-羟基-5-己烯酸酯的开发:他汀侧链的关键中间体
HMG-CoA还原酶抑制剂他汀类药物是最有效和最畅销的降胆固醇药物之一。他汀类药物的使用大大延长了人们的生活,并改善了他们的生活质量。他汀类药物的更有效,立体选择性和可持续合成的开发一直是最重要的。在本研究中,通过筛选酮还原酶(KRED)和优化反应,我们成功地在中试规模上通过KRED-06催化了酮酯1a的高立体选择性还原,而无需添加外源NADP +,产生了3.21 kg对映体纯叔丁基([R)-3-羟基-5-己烯酸(([R ) - 2a中)(96.2%的收率,> 99.9%的对映体过量(ee))。这种新开发的生物催化工艺减轻了我们在第一代使用NaBH 4和(l)-酒石酸合成(R)-2a时使用的低温条件(−40°C)。加上我们先前建立bromocarbonate的合成图3a经由一釜非对映选择性羧化/的bromocyclization([R )-图2a,我们已经开发了一种新颖,实用的合成路线,以他汀类药物的侧链,具有很大的潜力可实施到工业生产他汀类药物的。
更新日期:2020-09-20
中文翻译:

实用的生物催化合成叔丁基(R)-3-羟基-5-己烯酸酯的开发:他汀侧链的关键中间体
HMG-CoA还原酶抑制剂他汀类药物是最有效和最畅销的降胆固醇药物之一。他汀类药物的使用大大延长了人们的生活,并改善了他们的生活质量。他汀类药物的更有效,立体选择性和可持续合成的开发一直是最重要的。在本研究中,通过筛选酮还原酶(KRED)和优化反应,我们成功地在中试规模上通过KRED-06催化了酮酯1a的高立体选择性还原,而无需添加外源NADP +,产生了3.21 kg对映体纯叔丁基([R)-3-羟基-5-己烯酸(([R ) - 2a中)(96.2%的收率,> 99.9%的对映体过量(ee))。这种新开发的生物催化工艺减轻了我们在第一代使用NaBH 4和(l)-酒石酸合成(R)-2a时使用的低温条件(−40°C)。加上我们先前建立bromocarbonate的合成图3a经由一釜非对映选择性羧化/的bromocyclization([R )-图2a,我们已经开发了一种新颖,实用的合成路线,以他汀类药物的侧链,具有很大的潜力可实施到工业生产他汀类药物的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号