当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oligosaccharide Binding and Thermostability of Two Related AA9 Lytic Polysaccharide Monooxygenases.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.biochem.0c00312 Tobias Tandrup 1 , Theodora Tryfona 2 , Kristian Erik Høpfner Frandsen 1, 3 , Katja Salomon Johansen 4 , Paul Dupree 2 , Leila Lo Leggio 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-20 , DOI: 10.1021/acs.biochem.0c00312 Tobias Tandrup 1 , Theodora Tryfona 2 , Kristian Erik Høpfner Frandsen 1, 3 , Katja Salomon Johansen 4 , Paul Dupree 2 , Leila Lo Leggio 1
Affiliation
|
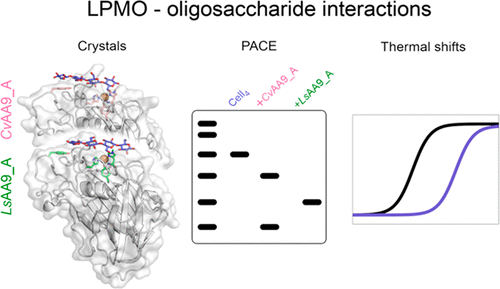
Lytic polysaccharide monooxygenases (LPMOs) are copper-dependent enzymes that cleave polysaccharide substrates oxidatively. First discovered because of their action on recalcitrant crystalline substrates (chitin and cellulose), a number of LPMOs are now reported to act on soluble substrates, including oligosaccharides. However, crystallographic complexes with oligosaccharides have been reported for only a single LPMO so far, an enzyme from the basidiomycete fungus Lentinus similis (LsAA9_A). Here we present a more detailed comparative study of LsAA9_A and an LPMO from the ascomycete fungus Collariella virescens (CvAA9_A) with which it shares 41.5% sequence identity. LsAA9_A is considerably more thermostable than CvAA9_A, and the structural basis for the difference has been investigated. We have compared the patterns of oligosaccharide cleavage and the patterns of binding in several new crystal structures explaining the basis for the product preferences of the two enzymes. Obtaining structural information about complexes of LPMOs with carbohydrates has proven to be very difficult in general judging from the structures reported in the literature thus far, and this can be attributed only partly to the low affinity for small substrates. We have thus evaluated the use of differential scanning fluorimetry as a guide to obtaining complex structures. Furthermore, an analysis of crystal packing of LPMOs and glycoside hydrolases corroborates the hypothesis that active site occlusion is a very significant problem for LPMO–substrate interaction analysis by crystallography, due to their relatively flat and extended substrate binding sites.
中文翻译:

寡糖结合和两个相关的AA9裂解多糖单加氧酶的热稳定性。
溶菌多糖单加氧酶(LPMO)是铜依赖性酶,可氧化裂解多糖底物。由于它们对顽固性结晶底物(几丁质和纤维素)的作用而首先被发现,据报道,许多LPMO对包括寡糖在内的可溶性底物起作用。然而,迄今为止,仅低水平的单晶磷酸二甲酯(LPMO),一种来自担子菌真菌小扁豆(Lintinus similis)的酶(Ls AA9_A),据报道与低聚糖形成的结晶复合物。在这里,我们提出了更详细的比较研究Ls AA9_A和来自子囊菌真菌天蛾Collariella virescens(Cv AA9_A)的LPMO,它具有41.5%的序列同一性。LsAA9_A比Cv热稳定得多AA9_A,并研究了差异的结构基础。我们已经比较了寡糖裂解的模式和几个新的晶体结构中的结合模式,解释了两种酶的产品偏好的基础。从迄今为止的文献报道的结构来看,一般来说很难获得有关LPMO与碳水化合物的复合物的结构信息,这只能部分归因于对小底物的低亲和力。因此,我们评估了使用差示扫描荧光法作为获得复杂结构的指南。此外,
更新日期:2020-09-15
中文翻译:

寡糖结合和两个相关的AA9裂解多糖单加氧酶的热稳定性。
溶菌多糖单加氧酶(LPMO)是铜依赖性酶,可氧化裂解多糖底物。由于它们对顽固性结晶底物(几丁质和纤维素)的作用而首先被发现,据报道,许多LPMO对包括寡糖在内的可溶性底物起作用。然而,迄今为止,仅低水平的单晶磷酸二甲酯(LPMO),一种来自担子菌真菌小扁豆(Lintinus similis)的酶(Ls AA9_A),据报道与低聚糖形成的结晶复合物。在这里,我们提出了更详细的比较研究Ls AA9_A和来自子囊菌真菌天蛾Collariella virescens(Cv AA9_A)的LPMO,它具有41.5%的序列同一性。LsAA9_A比Cv热稳定得多AA9_A,并研究了差异的结构基础。我们已经比较了寡糖裂解的模式和几个新的晶体结构中的结合模式,解释了两种酶的产品偏好的基础。从迄今为止的文献报道的结构来看,一般来说很难获得有关LPMO与碳水化合物的复合物的结构信息,这只能部分归因于对小底物的低亲和力。因此,我们评估了使用差示扫描荧光法作为获得复杂结构的指南。此外,


























 京公网安备 11010802027423号
京公网安备 11010802027423号