Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-21 , DOI: 10.1016/j.tet.2020.131520 Yan Shu , Jia-Peng Wang , Xue-Yun Cai , Xiao-Lan Li , Jun-Tao Hu , Cheng-Tong Sun , Le Cai , Zhong-Tao Ding
|
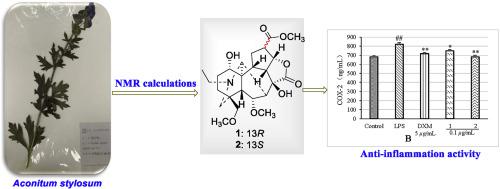
Two previously undescribed seco-type C19-diterpenoid alkaloids, stylosines A and B (1–2), which possess an unprecedented opened D ring by the C(15)–C(16) broken bond forming a five-membered C(15)−O–C(14) lactone ring, together with 14 known diterpenoid alkaloids were isolated from the roots of Aconitum stylosum (Ranunculaceae). Compound 1 is the first reported C19-diterpenoid alkaloid with an α-orientation at H-13. Their structures were determined by extensive spectroscopic analyses, NMR calculations and DP4+ analysis. A plausible biosynthetic pathway of the undescribed compounds was proposed. Compounds 1 and 2 showed significant inhibitory activity against the production of inflammatory cytokines (interleukin 1β, cyclooxygenase-2, and tumor necrosis factor α) in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages. Compounds 1 and 2 exhibited antibacterial activity against Staphylococcus aureus with MIC values of 2.00 and 32.00 μg/mL, respectively.
中文翻译:

stylosines A和B,来自乌头的抗炎性二萜类生物碱
两个以前未描述开环- C型19种-diterpenoid生物碱,stylosines A和B(1 - 2),其由C具有前所未有的打开d环(15) -C (16)断裂键形成五元C- (15 ) -O-C (14)内酯环与14种已知的二萜类生物碱是从铁乌头(毛an科)的根中分离出来的。化合物1是第一个报道的具有α的C 19-二萜生物碱-在H-13定向。它们的结构通过广泛的光谱分析,NMR计算和DP4 +分析确定。提出了未描述化合物的可能的生物合成途径。化合物1和2在脂多糖(LPS)诱导的RAW 264.7巨噬细胞中对炎症细胞因子(白介素1β,环氧合酶2和肿瘤坏死因子α)的产生具有显着的抑制活性。化合物1和2表现出抗菌活性对金黄色葡萄球菌为2.00和32.00 MIC值 μ分别克/毫升,。



























 京公网安备 11010802027423号
京公网安备 11010802027423号