Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-08-21 , DOI: 10.1016/j.bbamem.2020.183449 Isabela P Gomes 1 , Talita L Santos 1 , Amanda N de Souza 1 , Lúcio O Nunes 1 , Gabriele A Cardoso 2 , Carolina O Matos 3 , Lívia M F Costa 1 , Luciano M Lião 3 , Jarbas M Resende 4 , Rodrigo M Verly 1
|
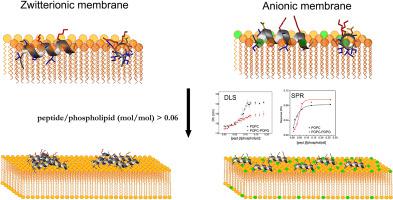
Studies have suggested that antimicrobial peptides act by different mechanisms, such as micellisation, self-assembly of nanostructures and pore formation on the membrane surface. This work presents an extensive investigation of the membrane interactions of the 14 amino-acid antimicrobial peptide hylaseptin P1-NH2 (HSP1-NH2), derived from the tree-frog Hyla punctata, which has stronger antifungal than antibacterial potential. Biophysical and structural analyses were performed and the correlated results were used to describe in detail the interactions of HSP1-NH2 with zwitterionic and anionic detergent micelles and phospholipid vesicles. HSP1-NH2 presents similar well-defined helical conformations in both zwitterionic and anionic micelles, although NMR spectroscopy revealed important structural differences in the peptide N-terminus. 2H exchange experiments of HSP1-NH2 indicated the insertion of the most N-terminal residues (1–3) in the DPC-d38 micelles. A higher enthalpic contribution was verified for the interaction of the peptide with anionic vesicles in comparison with zwitterionic vesicles. The pore formation ability of HSP1-NH2 (examined by dye release assays) and its effect on the size and surface charge as well as on the lipid acyl chain ordering (evaluated by Fourier-transform infrared spectroscopy) of anionic phospholipid vesicles showed membrane disruption even at low peptide-to-phospholipid ratios, and the effect increases proportionately to the peptide concentration. On the other hand, these biophysical investigations showed that a critical peptide-to-phospholipid ratio around 0.6 is essential for promoting disruption of zwitterionic membranes. In conclusion, this study demonstrates that the binding process of the antimicrobial HSP1-NH2 peptide depends on the membrane composition and peptide concentration.
中文翻译:

阿努兰抗菌肽HSP1-NH2的膜相互作用:与阴离子和两性离子仿生系统缔合的不同方面。
研究表明,抗菌肽通过不同的机制起作用,例如胶束化,纳米结构的自组装和膜表面上的孔形成。这项工作提出了对14种氨基酸抗菌肽hyepteptin P1-NH 2(HSP1-NH 2)的膜相互作用的广泛研究,这种抗菌作用源自树蛙Hyla punctata,其抗真菌能力强于抗菌潜能。进行了生物物理和结构分析,并使用相关结果详细描述了HSP1-NH 2与两性离子和阴离子去污剂胶束和磷脂囊泡的相互作用。HSP1-NH 2在两性离子和阴离子胶束中均显示出相似的定义良好的螺旋构象,尽管NMR光谱显示肽N端存在重要的结构差异。HSP1-NH 2的2 H交换实验表明,在DPC- d 38胶束中插入了最多N端残基(1-3)。与两性离子囊泡相比,该肽与阴离子囊泡的相互作用被证实具有更高的焓贡献。HSP1-NH 2的成孔能力(通过染料释放分析检查)及其对阴离子磷脂囊泡的大小和表面电荷以及脂酰基链有序性(通过傅里叶变换红外光谱法评估)的影响,即使在低的肽与磷脂比率下也显示出膜破裂。 ,效果随肽浓度成比例增加。另一方面,这些生物物理研究表明,关键的肽与磷脂之比约为0.6对于促进两性离子膜的破坏至关重要。总而言之,这项研究表明抗菌HSP1-NH 2肽的结合过程取决于膜的组成和肽的浓度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号