当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NBS-activated cross-dehydrogenative esterification of carboxylic acids with DMSO
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0qo00617c Ya Wu 1, 2, 3, 4 , Mengsha Zhang 1, 2, 3, 4 , Yanli Zhang 1, 2, 3, 4 , Mingyang Li 1, 2, 3, 4 , Weisheng Feng 1, 2, 3, 4 , Xiaoke Zheng 1, 2, 3, 4 , Lin Tang 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0qo00617c Ya Wu 1, 2, 3, 4 , Mengsha Zhang 1, 2, 3, 4 , Yanli Zhang 1, 2, 3, 4 , Mingyang Li 1, 2, 3, 4 , Weisheng Feng 1, 2, 3, 4 , Xiaoke Zheng 1, 2, 3, 4 , Lin Tang 4, 5, 6, 7
Affiliation
|
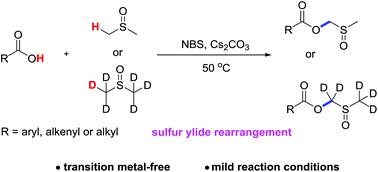
The first N-bromosuccinimide (NBS)-promoted transition metal-free cross-dehydrogenative esterification of carboxylic acids with dimethyl sulfoxide (DMSO) has been disclosed for formal C–O formation via Pummerer-type rearrangement. These transformations employ readily available carboxylic acids as the nucleophile and DMSO as the sulfur-containing carbon source and solvent under mild conditions, affording valuable (methylsulfinyl)methyl esters with a broad substrate scope. Furthermore, the developed protocol provides an efficient and straightforward method for the preparation of deuterated (methylsulfinyl)methyl esters by use of deuterium-labelled DMSO as the reagent.
中文翻译:

NBS活化的DMSO对羧酸的交叉脱氢酯化反应
首次公开了N-溴琥珀酰亚胺(NBS)促进的羧酸与二甲基亚砜(DMSO)的无过渡金属交叉脱氢酯化反应,可通过Pummerer型重排形成正式的C-O 。这些转化在温和的条件下采用容易获得的羧酸作为亲核试剂,并使用DMSO作为含硫的碳源和溶剂,从而提供了具有宽泛底物范围的有价值的(甲基亚磺酰基)甲基酯。此外,通过使用氘标记的DMSO作为试剂,开发的方案为制备氘代(甲基亚磺酰基)甲酯提供了一种有效而直接的方法。
更新日期:2020-09-16
中文翻译:

NBS活化的DMSO对羧酸的交叉脱氢酯化反应
首次公开了N-溴琥珀酰亚胺(NBS)促进的羧酸与二甲基亚砜(DMSO)的无过渡金属交叉脱氢酯化反应,可通过Pummerer型重排形成正式的C-O 。这些转化在温和的条件下采用容易获得的羧酸作为亲核试剂,并使用DMSO作为含硫的碳源和溶剂,从而提供了具有宽泛底物范围的有价值的(甲基亚磺酰基)甲基酯。此外,通过使用氘标记的DMSO作为试剂,开发的方案为制备氘代(甲基亚磺酰基)甲酯提供了一种有效而直接的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号