当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
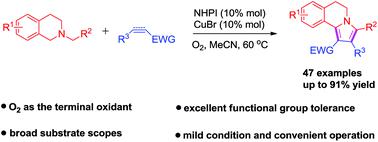
CuBr/NHPI co-catalyzed aerobic oxidative [3 + 2] cycloaddition-aromatization to access 5,6-dihydro-pyrrolo[2,1-a]isoquinolines.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0ob01403f Zhiyu Xie 1 , Fei Li , Liangfeng Niu , Hongbing Li , Jincai Zheng , Ruijing Han , Zhiyu Ju , Shanshan Li , Dandan Li
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-08-20 , DOI: 10.1039/d0ob01403f Zhiyu Xie 1 , Fei Li , Liangfeng Niu , Hongbing Li , Jincai Zheng , Ruijing Han , Zhiyu Ju , Shanshan Li , Dandan Li
Affiliation
|
An efficient and enviromentally friendly CuBr/NHPI co-catalyzed aerobic oxidative [3 + 2] cycloaddition–aromatization cascade was realized with N-substituted tetrahydroisoquinolines and electron-deficient olefins. Under the mild conditions, the reaction proceeded smoothly and displayed excellent functional group tolerance, affording 5,6-dihydro-pyrrolo[2,1-a]isoquinolines in good to high yields. This protocol exhibits a broad substrate scope to both N-alkyl tetrahydroisoquinolines and dipolarophile substrates.
中文翻译:

CuBr/NHPI 共催化需氧氧化 [3 + 2] 环加成-芳构化得到 5,6-二氢吡咯并 [2,1-a] 异喹啉。
用N-取代的四氢异喹啉和缺电子烯烃实现了高效且环保的CuBr/NHPI共催化好氧氧化[3 + 2]环加成-芳构化级联。在温和条件下,反应顺利进行,并表现出优异的官能团耐受性,以良好至高收率得到5,6-二氢-吡咯并[2,1- a ]异喹啉。该协议对N-烷基四氢异喹啉和亲偶极底物都表现出广泛的底物范围。
更新日期:2020-09-16
中文翻译:

CuBr/NHPI 共催化需氧氧化 [3 + 2] 环加成-芳构化得到 5,6-二氢吡咯并 [2,1-a] 异喹啉。
用N-取代的四氢异喹啉和缺电子烯烃实现了高效且环保的CuBr/NHPI共催化好氧氧化[3 + 2]环加成-芳构化级联。在温和条件下,反应顺利进行,并表现出优异的官能团耐受性,以良好至高收率得到5,6-二氢-吡咯并[2,1- a ]异喹啉。该协议对N-烷基四氢异喹啉和亲偶极底物都表现出广泛的底物范围。


























 京公网安备 11010802027423号
京公网安备 11010802027423号