当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of carbonate electrolytes on interaction of quinolone drug with anionic surfactant at various temperatures: A conductometric study
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-08-16 , DOI: 10.1002/poc.4121 Shamim Mahbub 1 , Mohayminul Islam 1 , Abuzayed Masum 1 , Parul Akter 1 , Md. Anamul Hoque 2 , Dileep Kumar 3, 4 , Farah Khan 5 , Malik Abdul Rub 6, 7 , Sulaiman Y.M. Alfaifi 6 , Abdullah M. Asiri 6, 7
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-08-16 , DOI: 10.1002/poc.4121 Shamim Mahbub 1 , Mohayminul Islam 1 , Abuzayed Masum 1 , Parul Akter 1 , Md. Anamul Hoque 2 , Dileep Kumar 3, 4 , Farah Khan 5 , Malik Abdul Rub 6, 7 , Sulaiman Y.M. Alfaifi 6 , Abdullah M. Asiri 6, 7
Affiliation
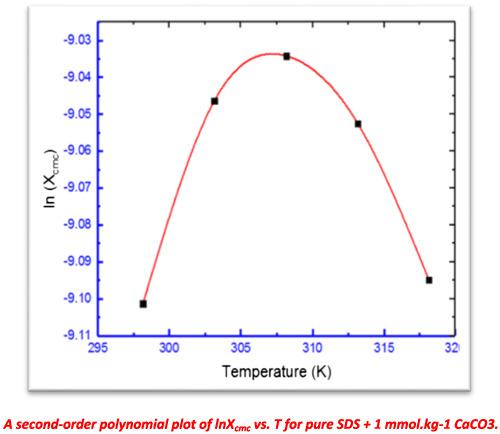
|
The role of different carbonate salts on the interaction of anionic surfactant sodium dodecyl sulfate (SDS) with employed quinolone antibiotic drug (ciprofloxacin hydrochloride [CFH]) was explosively inspected through measuring specific conductivity (κ) of the investigated system. The drug, CFH, is an excellent antibiotic for the treatment of different bacterial infections like skin, urinary tract, and respiratory infections. The estimated critical micelle concentrations (cmc) of SDS were observed to be dwindled in the attendance of electrolytes and cmc reduction continues with the escalation of the electrolyte's concentration. The magnitudes of cmc for the SDS + CFH mixture were reduced with the elevation of temperature in K2CO3 and CaCO3 medium while increased with the rise of the temperature in the Na2CO3 medium. The magnitudes of counterion binding to micelles (β) were increased with the increase of the concentration of Na2CO3 and K2CO3 up to definite value and then reduced with the increase of successive rise of concentration of both salts at all temperatures while decreased monotonically with the rise of electrolyte concentration in CaCO3 medium. The values of standard free energy (
 ) were achieved to be negative in each case suggesting spontaneous self‐assembly formation. The standard entropy change (
) were achieved to be negative in each case suggesting spontaneous self‐assembly formation. The standard entropy change (
 ) along with enthalpy change (
) along with enthalpy change (
 ) was also assessed and discussed elaborately.
) was also assessed and discussed elaborately.
中文翻译:

碳酸盐电解质在不同温度下对喹诺酮药物与阴离子表面活性剂相互作用的作用:电导研究
通过测量所研究系统的比电导率(κ),爆炸性地检查了不同碳酸盐对阴离子表面活性剂十二烷基硫酸钠(SDS)与使用的喹诺酮抗生素药物(环丙沙星盐酸盐[CFH])相互作用的作用。CFH药物是治疗各种细菌感染(如皮肤,泌尿道和呼吸道感染)的极好的抗生素。观察到SDS的估计临界胶束浓度(cmc)随电解质的增加而减少,并且随着电解质浓度的升高,cmc的降低继续。SDS + CFH混合物的cmc值随K的温度升高而降低2 CO 3和CaCO 3中,同时与温度的以Na升高而增加2 CO 3介质。Na 2 CO 3和K 2 CO 3的浓度升高到一定值时,反离子与胶束(β)的结合强度增大,然后在两种温度下随着两种盐浓度的连续升高而减小。随着CaCO 3介质中电解质浓度的升高单调下降。标准自由能的值( )在每种情况下都为负,表明自发形成自组装。还对标准熵变(
)在每种情况下都为负,表明自发形成自组装。还对标准熵变(
 )和焓变(
)和焓变(
 )进行了评估和详细讨论。
)进行了评估和详细讨论。
更新日期:2020-08-16
 ) were achieved to be negative in each case suggesting spontaneous self‐assembly formation. The standard entropy change (
) were achieved to be negative in each case suggesting spontaneous self‐assembly formation. The standard entropy change (
 ) along with enthalpy change (
) along with enthalpy change (
 ) was also assessed and discussed elaborately.
) was also assessed and discussed elaborately.
中文翻译:

碳酸盐电解质在不同温度下对喹诺酮药物与阴离子表面活性剂相互作用的作用:电导研究
通过测量所研究系统的比电导率(κ),爆炸性地检查了不同碳酸盐对阴离子表面活性剂十二烷基硫酸钠(SDS)与使用的喹诺酮抗生素药物(环丙沙星盐酸盐[CFH])相互作用的作用。CFH药物是治疗各种细菌感染(如皮肤,泌尿道和呼吸道感染)的极好的抗生素。观察到SDS的估计临界胶束浓度(cmc)随电解质的增加而减少,并且随着电解质浓度的升高,cmc的降低继续。SDS + CFH混合物的cmc值随K的温度升高而降低2 CO 3和CaCO 3中,同时与温度的以Na升高而增加2 CO 3介质。Na 2 CO 3和K 2 CO 3的浓度升高到一定值时,反离子与胶束(β)的结合强度增大,然后在两种温度下随着两种盐浓度的连续升高而减小。随着CaCO 3介质中电解质浓度的升高单调下降。标准自由能的值(
 )在每种情况下都为负,表明自发形成自组装。还对标准熵变(
)在每种情况下都为负,表明自发形成自组装。还对标准熵变(
 )和焓变(
)和焓变(
 )进行了评估和详细讨论。
)进行了评估和详细讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号