当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lewis acids and bases as molecular dopants for organic semiconductors
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-05-05 , DOI: 10.1002/poc.4077 Colin R. Bridges 1 , Thomas Baumgartner 1
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2020-05-05 , DOI: 10.1002/poc.4077 Colin R. Bridges 1 , Thomas Baumgartner 1
Affiliation
|
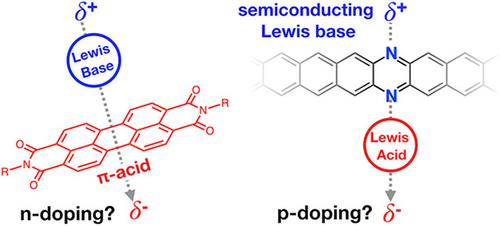
Controlling the concentration of charge carriers (mobile electrons and holes) in organic semiconductors is vital to precisely controlling their electronic properties. Significant efforts have gone into understanding how molecular dopants induce charge carriers in organic semiconductors. The most widely used doping mechanisms occur via electron transfer (i.e., oxidation or reduction of the semiconductor) or via reaction with a strong Brønsted acid. Recently, strong Lewis acids have been observed to induce p‐type charge carriers in organic semiconductors with greater efficiency than classical dopants. The mechanism of Lewis‐acid doping could not easily be unified with either classical doping methods and has been under intense scrutiny over the past 5 years. Very recently, the Lewis‐acid doping effects have been shown to be due to water impurities in commercial Lewis acids forming strong Brønsted acids. This means that many studies on doping using Lewis acids may be occurring via a Brønsted‐acid doping mechanism. This recent revelation explains some observations in literature, but not all, and there are still unanswered questions. The nature of the Lewis acid and organic semiconductor can significantly impact the doping mechanism and the doping efficiency. Additionally, strong evidence for alternative doping mechanisms using Lewis acids not involving water has been shown. Lewis‐acid doping has mostly been studied as a p‐type dopant method on Lewis‐basic polymers. There is growing literature showing Lewis bases can also act as n‐type dopants, excluding Brønsted‐acid doping as a possible mechanism. In this tutorial review, we will present a brief overview on molecular doping of organic semiconductors, survey the literature on p‐type and n‐type Lewis doping, outline several proposed mechanisms, and speculate on some possible mechanisms using literature observations.
中文翻译:

路易斯酸和碱作为有机半导体的分子掺杂剂
控制有机半导体中载流子(移动电子和空穴)的浓度对于精确控制其电子性能至关重要。在了解分子掺杂剂如何在有机半导体中诱导电荷载流子方面已经付出了巨大的努力。最广泛使用的掺杂机制是通过电子转移(即半导体的氧化或还原)或通过与强布朗斯台德酸的反应发生的。最近,已观察到强路易斯酸比传统的掺杂剂能更有效地诱导有机半导体中的p型电荷载流子。路易斯酸掺杂的机理很难通过任何一种经典掺杂方法来统一,并且在过去的5年中受到了严格的审查。最近,路易斯酸的掺杂作用已被证明是由于商业路易斯酸中的水杂质形成了强布朗斯台德酸。这意味着许多关于路易斯酸掺杂的研究可能是通过布朗斯台德酸掺杂机理进行的。最近的这个发现解释了文学中的一些观察,但不是全部,仍然有未解决的问题。路易斯酸和有机半导体的性质会显着影响掺杂机理和掺杂效率。另外,已经显示出使用不涉及水的路易斯酸来替代掺杂机制的有力证据。对路易斯酸掺杂的研究主要是针对路易斯碱性聚合物的ap型掺杂方法。越来越多的文献表明,路易斯碱也可以用作n型掺杂剂,但不包括布朗斯台德酸掺杂是一种可能的机制。
更新日期:2020-05-05
中文翻译:

路易斯酸和碱作为有机半导体的分子掺杂剂
控制有机半导体中载流子(移动电子和空穴)的浓度对于精确控制其电子性能至关重要。在了解分子掺杂剂如何在有机半导体中诱导电荷载流子方面已经付出了巨大的努力。最广泛使用的掺杂机制是通过电子转移(即半导体的氧化或还原)或通过与强布朗斯台德酸的反应发生的。最近,已观察到强路易斯酸比传统的掺杂剂能更有效地诱导有机半导体中的p型电荷载流子。路易斯酸掺杂的机理很难通过任何一种经典掺杂方法来统一,并且在过去的5年中受到了严格的审查。最近,路易斯酸的掺杂作用已被证明是由于商业路易斯酸中的水杂质形成了强布朗斯台德酸。这意味着许多关于路易斯酸掺杂的研究可能是通过布朗斯台德酸掺杂机理进行的。最近的这个发现解释了文学中的一些观察,但不是全部,仍然有未解决的问题。路易斯酸和有机半导体的性质会显着影响掺杂机理和掺杂效率。另外,已经显示出使用不涉及水的路易斯酸来替代掺杂机制的有力证据。对路易斯酸掺杂的研究主要是针对路易斯碱性聚合物的ap型掺杂方法。越来越多的文献表明,路易斯碱也可以用作n型掺杂剂,但不包括布朗斯台德酸掺杂是一种可能的机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号