当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diversity‐oriented Functionalization of Cyclodienes Through Selective Cycloaddition/Ring‐opening/Cross‐metathesis Protocols; Transformation of a “Flatland” into Three‐dimensional Scaffolds With Stereo‐ and Regiocontrol
The Chemical Record ( IF 6.6 ) Pub Date : 2020-07-28 , DOI: 10.1002/tcr.202000070 Loránd Kiss 1, 2 , Zsanett Benke 1, 2 , Attila M. Remete 1, 2 , Ferenc Fülöp 1, 2, 3
The Chemical Record ( IF 6.6 ) Pub Date : 2020-07-28 , DOI: 10.1002/tcr.202000070 Loránd Kiss 1, 2 , Zsanett Benke 1, 2 , Attila M. Remete 1, 2 , Ferenc Fülöp 1, 2, 3
Affiliation
|
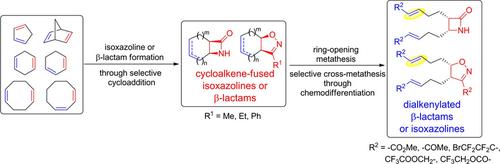
This article presents selective transformations of some readily available cyclodienes through simple chemical procedures into novel functionalized small‐molecular entities. The syntheses hereby described involved selective cycloadditions, followed by ring‐opening metathesis of the resulting β‐lactam or isoxazoline derivatives and selective cross‐metathesis by differentiation of the olefin bonds on the alkenylated heterocycles. The cross‐metathesis transformations have been detailed, which were performed under various experimental conditions with the aim of exploring chemodiscrimination of the olefin bonds and delivering the corresponding functionalized β‐lactam or isoxazoline derivatives.
中文翻译:

通过选择性的环加成/开环/交叉易位方案,以多样性为导向的环二烯官能化;通过立体和区域控制将“平台”转变为三维支架
本文介绍了通过简单的化学程序将一些易于获得的环二烯选择性转化为新型功能化的小分子实体的方法。此处描述的合成涉及选择性环加成反应,然后是所得β-内酰胺或异恶唑啉衍生物的开环复分解,以及通过烯基化杂环上烯烃键的区分进行选择性交叉复分解。详细介绍了跨复分解转化,这些转化是在各种实验条件下进行的,目的是探索烯烃键的化学歧化作用并提供相应的官能化β-内酰胺或异恶唑啉衍生物。
更新日期:2020-07-28
中文翻译:

通过选择性的环加成/开环/交叉易位方案,以多样性为导向的环二烯官能化;通过立体和区域控制将“平台”转变为三维支架
本文介绍了通过简单的化学程序将一些易于获得的环二烯选择性转化为新型功能化的小分子实体的方法。此处描述的合成涉及选择性环加成反应,然后是所得β-内酰胺或异恶唑啉衍生物的开环复分解,以及通过烯基化杂环上烯烃键的区分进行选择性交叉复分解。详细介绍了跨复分解转化,这些转化是在各种实验条件下进行的,目的是探索烯烃键的化学歧化作用并提供相应的官能化β-内酰胺或异恶唑啉衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号