当前位置:
X-MOL 学术
›
Polym. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Network formation by aza‐Michael addition of primary amines to vinyl end groups of enzymatically synthesized poly(glycerol adipate)
Polymer International ( IF 3.2 ) Pub Date : 2020-08-19 , DOI: 10.1002/pi.6102 Razan Alaneed 1, 2 , Yury Golitsyn 3 , Till Hauenschild 1 , Markus Pietzsch 2 , Detlef Reichert 3 , Jörg Kressler 1
Polymer International ( IF 3.2 ) Pub Date : 2020-08-19 , DOI: 10.1002/pi.6102 Razan Alaneed 1, 2 , Yury Golitsyn 3 , Till Hauenschild 1 , Markus Pietzsch 2 , Detlef Reichert 3 , Jörg Kressler 1
Affiliation
|
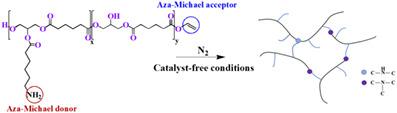
A highly efficient approach for the synthesis of polyester‐based networks via aza‐Michael addition of primary amines to α,β‐unsaturated (vinyl) end groups of poly(glycerol adipate) (PGA) was achieved. By acylation of PGA with 6‐(Fmoc‐amino)hexanoic acid side chains via Steglich esterification, protected amine‐functionalized PGA was obtained. This was followed by the removal of fluorenylmethyloxycarbonyl (Fmoc) protecting groups and the synthesis of PGA‐based networks under catalyst‐free conditions. The successful conjugate addition of primary amines to vinyl end groups and network formation were confirmed using 13C magic angle spinning NMR and Fourier transform infrared spectroscopy. Network heterogeneity and defects were quantitatively investigated using 1H double‐quantum NMR spectroscopy. Finally, a hydrogel was prepared with potential biomedical applications.
中文翻译:

通过在酶促合成的聚己二酸甘油酯的乙烯基端基上添加伯胺的氮杂-迈克尔加成网络形成
通过在聚己二酸甘油酯(PGA)的α,β-不饱和(乙烯基)端基上氮杂伯胺的氮杂-迈克尔加成反应,实现了一种高效的聚酯基网络合成方法。通过Steglich酯化作用将PGA与6-(Fmoc-氨基)己酸侧链酰化,可获得受保护的胺官能化PGA。接下来是除去芴基甲氧羰基(Fmoc)保护基,并在无催化剂的条件下合成基于PGA的网络。使用13 C魔角旋转NMR和傅里叶变换红外光谱法证实了伯胺向乙烯基端基成功共轭加成和网络形成。网络异质性和缺陷使用1 H双量子NMR光谱。最后,制备了具有潜在生物医学应用的水凝胶。
更新日期:2020-08-19
中文翻译:

通过在酶促合成的聚己二酸甘油酯的乙烯基端基上添加伯胺的氮杂-迈克尔加成网络形成
通过在聚己二酸甘油酯(PGA)的α,β-不饱和(乙烯基)端基上氮杂伯胺的氮杂-迈克尔加成反应,实现了一种高效的聚酯基网络合成方法。通过Steglich酯化作用将PGA与6-(Fmoc-氨基)己酸侧链酰化,可获得受保护的胺官能化PGA。接下来是除去芴基甲氧羰基(Fmoc)保护基,并在无催化剂的条件下合成基于PGA的网络。使用13 C魔角旋转NMR和傅里叶变换红外光谱法证实了伯胺向乙烯基端基成功共轭加成和网络形成。网络异质性和缺陷使用1 H双量子NMR光谱。最后,制备了具有潜在生物医学应用的水凝胶。


























 京公网安备 11010802027423号
京公网安备 11010802027423号