当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tau-induced upregulation of C/EBPβ-TRPC1-SOCE signaling aggravates tauopathies: A vicious cycle in Alzheimer neurodegeneration.
Aging Cell ( IF 7.8 ) Pub Date : 2020-08-20 , DOI: 10.1111/acel.13209 Jinwang Ye 1 , Ying Yin 1 , Yaling Yin 2 , Huaqiu Zhang 3 , Huali Wan 1 , Lu Wang 2 , Yue Zuo 2 , Di Gao 1 , Mengzhu Li 1, 4 , Jun Li 4 , Yanchao Liu 1 , Dan Ke 1 , Jian-Zhi Wang 1, 5
Aging Cell ( IF 7.8 ) Pub Date : 2020-08-20 , DOI: 10.1111/acel.13209 Jinwang Ye 1 , Ying Yin 1 , Yaling Yin 2 , Huaqiu Zhang 3 , Huali Wan 1 , Lu Wang 2 , Yue Zuo 2 , Di Gao 1 , Mengzhu Li 1, 4 , Jun Li 4 , Yanchao Liu 1 , Dan Ke 1 , Jian-Zhi Wang 1, 5
Affiliation
|
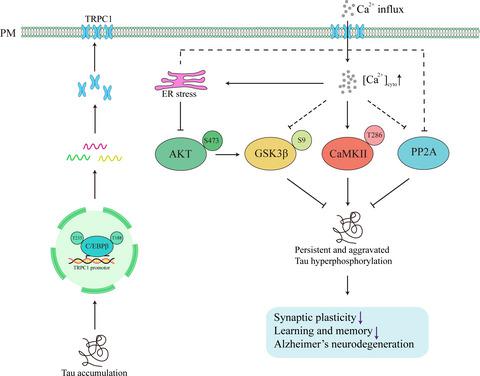
Intracellular accumulating of the hyperphosphorylated tau plays a pivotal role in neurodegeneration of Alzheimer disease (AD), but the mechanisms underlying the gradually aggravated tau hyperphosphorylation remain elusive. Here, we show that increasing intracellular tau could upregulate mRNA and protein levels of TRPC1 (transient receptor potential channel 1) with an activated store‐operated calcium entry (SOCE), an increased intraneuronal steady‐state [Ca2+]i, an enhanced endoplasmic reticulum (ER) stress, an imbalanced protein kinases and phosphatase, and an aggravated tauopathy. Furthermore, overexpressing TRPC1 induced ER stress, kinases‐phosphatase imbalance, tau hyperphosphorylation and cognitive deficits in cultured neurons and mice, while pharmacological inhibiting or knockout TRPC1 attenuated the hTau‐induced deregulations in SOCE, ER homeostasis, kinases‐phosphatase balance, and tau phosphorylation level with improved synaptic and cognitive functions. Finally, an increased CCAAT‐enhancer‐binding protein (C/EBPβ) activity was observed in hTau‐overexpressing cells and the hippocampus of the AD patients, while downregulating C/EBPβ by siRNA abolished the hTau‐induced TRPC1 upregulation. These data reveal that increasing intracellular tau can upregulate C/EBPβ‐TRPC1‐SOCE signaling and thus disrupt phosphorylating system, which together aggravates tau pathologies leading to a chronic neurodegeneration.
中文翻译:

Tau 诱导的 C/EBPβ-TRPC1-SOCE 信号上调加剧了 tauopathies:阿尔茨海默病神经退行性疾病的恶性循环。
过度磷酸化 tau 的细胞内积累在阿尔茨海默病 (AD) 的神经变性中起着关键作用,但逐渐加重的 tau 过度磷酸化的机制仍然难以捉摸。在这里,我们表明增加细胞内 tau 可以上调 TRPC1(瞬时受体电位通道 1)的 mRNA 和蛋白质水平,激活钙池操纵钙进入(SOCE),增加神经元内稳态 [Ca 2+ ] i、增强的内质网 (ER) 应激、不平衡的蛋白激酶和磷酸酶以及加重的 tau 蛋白病变。此外,在培养的神经元和小鼠中,过表达 TRPC1 诱导 ER 应激、激酶-磷酸酶失衡、tau 过度磷酸化和认知缺陷,而药理学抑制或敲除 TRPC1 减弱了 hTau 诱导的 SOCE、ER 稳态、激酶-磷酸酶平衡和 tau 磷酸化的失调水平与改善的突触和认知功能。最后,在 hTau 过表达细胞和 AD 患者的海马中观察到 CCAAT 增强子结合蛋白(C/EBPβ)活性增加,而通过 siRNA 下调 C/EBPβ 消除了 hTau 诱导的 TRPC1 上调。
更新日期:2020-09-24
中文翻译:

Tau 诱导的 C/EBPβ-TRPC1-SOCE 信号上调加剧了 tauopathies:阿尔茨海默病神经退行性疾病的恶性循环。
过度磷酸化 tau 的细胞内积累在阿尔茨海默病 (AD) 的神经变性中起着关键作用,但逐渐加重的 tau 过度磷酸化的机制仍然难以捉摸。在这里,我们表明增加细胞内 tau 可以上调 TRPC1(瞬时受体电位通道 1)的 mRNA 和蛋白质水平,激活钙池操纵钙进入(SOCE),增加神经元内稳态 [Ca 2+ ] i、增强的内质网 (ER) 应激、不平衡的蛋白激酶和磷酸酶以及加重的 tau 蛋白病变。此外,在培养的神经元和小鼠中,过表达 TRPC1 诱导 ER 应激、激酶-磷酸酶失衡、tau 过度磷酸化和认知缺陷,而药理学抑制或敲除 TRPC1 减弱了 hTau 诱导的 SOCE、ER 稳态、激酶-磷酸酶平衡和 tau 磷酸化的失调水平与改善的突触和认知功能。最后,在 hTau 过表达细胞和 AD 患者的海马中观察到 CCAAT 增强子结合蛋白(C/EBPβ)活性增加,而通过 siRNA 下调 C/EBPβ 消除了 hTau 诱导的 TRPC1 上调。



























 京公网安备 11010802027423号
京公网安备 11010802027423号