Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Allovalency observed by transferred NOE: interactions of sulfated tyrosine residues in the N‐terminal segment of CCR5 with the CCL5 chemokine
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-08-19 , DOI: 10.1111/febs.15503 Naama Kessler 1 , Sabine R Akabayov 1 , Adi Moseri 1 , Leah S Cohen 2, 3 , Damir Sakhapov 1 , David Bolton 4 , Brandon Fridman 2 , Lewis E Kay 5, 6, 7, 8 , Fred Naider 2, 3 , Jacob Anglister 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-08-19 , DOI: 10.1111/febs.15503 Naama Kessler 1 , Sabine R Akabayov 1 , Adi Moseri 1 , Leah S Cohen 2, 3 , Damir Sakhapov 1 , David Bolton 4 , Brandon Fridman 2 , Lewis E Kay 5, 6, 7, 8 , Fred Naider 2, 3 , Jacob Anglister 1
Affiliation
|
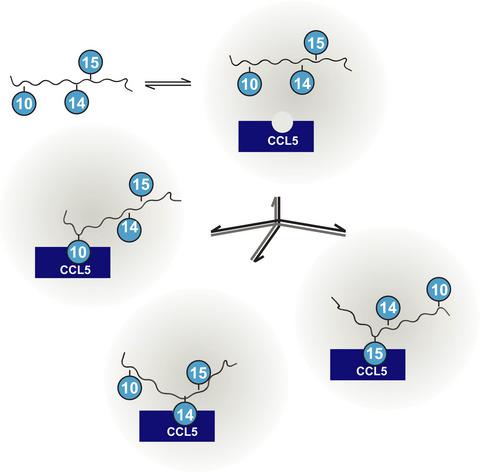
The N‐terminal segment of the chemokine receptor Human CC chemokine receptor 5 (CCR5), Nt‐CCR5, contains four tyrosine residues, Y3, Y10, Y14, and Y15. Sulfation of at least two of these tyrosine residues was found to be essential for high‐affinity binding of CCR5 to its chemokine ligands. Here, we show that among the monosulfated Nt‐CCR5(8‐20) peptide surrogates (sNt‐CCR5) those sulfated at Y15 and Y14 have the highest affinity for the CC chemokine ligand 5 (CCL5) chemokine in comparison with monosulfation at position Y10. Sulfation at Y3 was not investigated. A peptide sulfated at both Y14 and Y15 has the highest affinity for CCL5 by up to a factor of 3, in comparison with the other disulfated (sNt‐CCR5) peptides. Chemical shift perturbation analysis and transferred nuclear Overhauser effect measurements indicate that the sulfated tyrosine residues interact with the same CCL5‐binding pocket and that each of the sulfated tyrosines at positions 10, 14, and 15 can occupy individually the binding site on CCL5 in a similar manner, although with somewhat different affinity, suggesting the possibility of allovalency in sulfated Nt‐CCR5 peptides. The affinity of the disulfated peptides to CCL5 could be increased by this allovalency and by stronger electrostatic interactions.
中文翻译:

通过转移的NOE观察到的同化价:CCR5 N末端区段中的硫酸化酪氨酸残基与CCL5趋化因子的相互作用
趋化因子受体人类CC趋化因子受体5(CCR5)的N末端片段Nt-CCR5包含四个酪氨酸残基Y3,Y10,Y14和Y15。发现至少两个酪氨酸残基的硫酸化对于CCR5与其趋化因子配体的高亲和力结合至关重要。在这里,我们表明在单硫酸盐化的Nt-CCR5(8-20)肽替代物中(sNt-CCR5)与在Y10位置的单硫酸盐化相比,在Y15和Y14处被硫酸化的那些对CC趋化因子配体5(CCL5)趋化因子的亲和力最高。 。没有研究Y 3的硫化。与其他二硫化(sNt-CCR5)肽相比,在Y14和Y15处被硫酸化的肽与CCL5的亲和性最高,最高可达3倍。化学位移扰动分析和转移的核Overhauser效应测量结果表明,硫酸化的酪氨酸残基与相同的CCL5结合口袋相互作用,并且第10、14和15位的每个硫酸化酪氨酸可以分别占据CCL5上的结合位点,类似尽管亲和力略有不同,但这种方式暗示了硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。提示硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。提示硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。
更新日期:2020-08-19
中文翻译:

通过转移的NOE观察到的同化价:CCR5 N末端区段中的硫酸化酪氨酸残基与CCL5趋化因子的相互作用
趋化因子受体人类CC趋化因子受体5(CCR5)的N末端片段Nt-CCR5包含四个酪氨酸残基Y3,Y10,Y14和Y15。发现至少两个酪氨酸残基的硫酸化对于CCR5与其趋化因子配体的高亲和力结合至关重要。在这里,我们表明在单硫酸盐化的Nt-CCR5(8-20)肽替代物中(sNt-CCR5)与在Y10位置的单硫酸盐化相比,在Y15和Y14处被硫酸化的那些对CC趋化因子配体5(CCL5)趋化因子的亲和力最高。 。没有研究Y 3的硫化。与其他二硫化(sNt-CCR5)肽相比,在Y14和Y15处被硫酸化的肽与CCL5的亲和性最高,最高可达3倍。化学位移扰动分析和转移的核Overhauser效应测量结果表明,硫酸化的酪氨酸残基与相同的CCL5结合口袋相互作用,并且第10、14和15位的每个硫酸化酪氨酸可以分别占据CCL5上的结合位点,类似尽管亲和力略有不同,但这种方式暗示了硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。提示硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。提示硫酸化Nt-CCR5肽具有同化作用的可能性。通过这种同化作用和更强的静电相互作用,可以增加二硫化肽对CCL5的亲和力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号