Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ca2+ -dependent release of ATP from astrocytes affects herpes simplex virus type 1 infection of neurons.
Glia ( IF 6.2 ) Pub Date : 2020-08-20 , DOI: 10.1002/glia.23895 Domenica Donatella Li Puma 1, 2 , Maria Elena Marcocci 3 , Giacomo Lazzarino 4 , Giovanna De Chiara 5 , Barbara Tavazzi 2, 6 , Anna Teresa Palamara 3, 7 , Roberto Piacentini 1, 2 , Claudio Grassi 1, 2
Glia ( IF 6.2 ) Pub Date : 2020-08-20 , DOI: 10.1002/glia.23895 Domenica Donatella Li Puma 1, 2 , Maria Elena Marcocci 3 , Giacomo Lazzarino 4 , Giovanna De Chiara 5 , Barbara Tavazzi 2, 6 , Anna Teresa Palamara 3, 7 , Roberto Piacentini 1, 2 , Claudio Grassi 1, 2
Affiliation
|
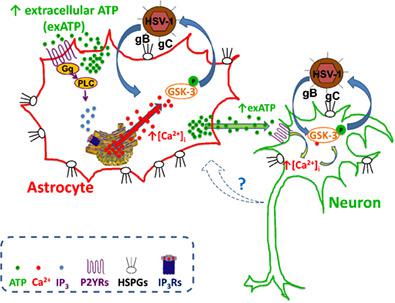
Astrocytes provide metabolic support for neurons and modulate their functions by releasing a plethora of neuroactive molecules diffusing to neighboring cells. Here we report that astrocytes also play a role in cortical neurons' vulnerability to Herpes simplex virus type‐1 (HSV‐1) infection through the release of extracellular ATP. We found that the interaction of HSV‐1 with heparan sulfate proteoglycans expressed on the plasma membrane of astrocytes triggered phospholipase C‐mediated IP3‐dependent intracellular Ca2+ transients causing extracellular release of ATP. ATP binds membrane purinergic P2 receptors (P2Rs) of both neurons and astrocytes causing an increase in intracellular Ca2+ concentration that activates the Glycogen Synthase Kinase (GSK)‐3β, whose action is necessary for HSV‐1 entry/replication in these cells. Indeed, in co‐cultures of neurons and astrocytes HSV‐1‐infected neurons were only found in proximity of infected astrocytes releasing ATP, whereas in the presence of fluorocitrate, an inhibitor of astrocyte metabolism, switching‐off the HSV‐1‐induced ATP release, very few neurons were infected. The addition of exogenous ATP, mimicking that released by astrocytes after HSV‐1 challenge, restored the ability of HSV‐1 to infect neurons co‐cultured with metabolically‐inhibited astrocytes. The ATP‐activated, P2R‐mediated, and GSK‐3‐dependent molecular pathway underlying HSV‐1 infection is likely shared by neurons and astrocytes, given that the blockade of either P2Rs or GSK‐3 activation inhibited infection of both cell types. These results add a new layer of information to our understanding of the critical role played by astrocytes in regulating neuronal functions and their response to noxious stimuli including microbial agents via Ca2+‐dependent release of neuroactive molecules.
中文翻译:

星形胶质细胞中 ATP 的 Ca2+ 依赖性释放影响神经元的 1 型单纯疱疹病毒感染。
星形胶质细胞为神经元提供代谢支持,并通过释放大量扩散到邻近细胞的神经活性分子来调节它们的功能。在这里,我们报告星形胶质细胞还通过释放细胞外 ATP 在皮质神经元对单纯疱疹病毒 1 型 (HSV-1) 感染的脆弱性中发挥作用。我们发现 HSV-1 与星形胶质细胞质膜上表达的硫酸乙酰肝素蛋白聚糖的相互作用触发了磷脂酶 C 介导的 IP 3依赖性细胞内 Ca 2+瞬变,导致细胞外释放 ATP。ATP 结合神经元和星形胶质细胞的膜嘌呤 P2 受体 (P2R),导致细胞内 Ca 2+增加激活糖原合酶激酶 (GSK)-3β 的浓度,其作用是 HSV-1 在这些细胞中进入/复制所必需的。事实上,在神经元和星形胶质细胞的共培养物中,HSV-1 感染的神经元仅在受感染的星形胶质细胞附近发现释放 ATP,而在星形胶质细胞代谢抑制剂氟柠檬酸盐的存在下,会关闭 HSV-1 诱导的 ATP释放,很少有神经元被感染。添加外源 ATP,模拟 HSV-1 攻击后星形胶质细胞释放的 ATP,恢复了 HSV-1 感染与代谢抑制的星形胶质细胞共培养的神经元的能力。HSV-1 感染的 ATP 激活、P2R 介导和 GSK-3 依赖性分子途径可能由神经元和星形胶质细胞共享,鉴于 P2Rs 或 GSK-3 激活的阻断抑制了两种细胞类型的感染。这些结果为我们理解星形胶质细胞在调节神经元功能及其对有害刺激(包括通过 Ca神经活性分子的2+依赖性释放。
更新日期:2020-08-20
中文翻译:

星形胶质细胞中 ATP 的 Ca2+ 依赖性释放影响神经元的 1 型单纯疱疹病毒感染。
星形胶质细胞为神经元提供代谢支持,并通过释放大量扩散到邻近细胞的神经活性分子来调节它们的功能。在这里,我们报告星形胶质细胞还通过释放细胞外 ATP 在皮质神经元对单纯疱疹病毒 1 型 (HSV-1) 感染的脆弱性中发挥作用。我们发现 HSV-1 与星形胶质细胞质膜上表达的硫酸乙酰肝素蛋白聚糖的相互作用触发了磷脂酶 C 介导的 IP 3依赖性细胞内 Ca 2+瞬变,导致细胞外释放 ATP。ATP 结合神经元和星形胶质细胞的膜嘌呤 P2 受体 (P2R),导致细胞内 Ca 2+增加激活糖原合酶激酶 (GSK)-3β 的浓度,其作用是 HSV-1 在这些细胞中进入/复制所必需的。事实上,在神经元和星形胶质细胞的共培养物中,HSV-1 感染的神经元仅在受感染的星形胶质细胞附近发现释放 ATP,而在星形胶质细胞代谢抑制剂氟柠檬酸盐的存在下,会关闭 HSV-1 诱导的 ATP释放,很少有神经元被感染。添加外源 ATP,模拟 HSV-1 攻击后星形胶质细胞释放的 ATP,恢复了 HSV-1 感染与代谢抑制的星形胶质细胞共培养的神经元的能力。HSV-1 感染的 ATP 激活、P2R 介导和 GSK-3 依赖性分子途径可能由神经元和星形胶质细胞共享,鉴于 P2Rs 或 GSK-3 激活的阻断抑制了两种细胞类型的感染。这些结果为我们理解星形胶质细胞在调节神经元功能及其对有害刺激(包括通过 Ca神经活性分子的2+依赖性释放。


























 京公网安备 11010802027423号
京公网安备 11010802027423号