当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of quinoline‐based hybrid as inhibitor of methionine aminopeptidase 1 from Leishmania donovani
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-08-20 , DOI: 10.1111/cbdd.13783 Saleem Yousuf Bhat 1 , Sonal Bhandari 2 , Pavitra Suresh Thacker 2 , Mohammed Arifuddin 2 , Insaf Ahmed Qureshi 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-08-20 , DOI: 10.1111/cbdd.13783 Saleem Yousuf Bhat 1 , Sonal Bhandari 2 , Pavitra Suresh Thacker 2 , Mohammed Arifuddin 2 , Insaf Ahmed Qureshi 1
Affiliation
|
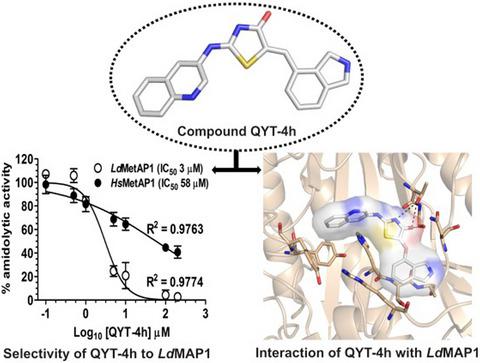
Methionine aminopeptidase 1 (MetAP1) is a target for drug discovery against many adversaries and a potential antileishmanial target for its role in N‐terminal methionine processing. As an effort towards new inhibitor discovery against methionine aminopeptidase 1 from Leishmania donovani (LdMetAP1), we have synthesized a series of quinoline‐based hybrids, that is (Z)‐5‐((Z)‐benzylidine)‐2‐(quinolin‐3‐ylimino)thiazolidin‐4‐ones (QYT‐4a‐i) whose in vitro screening led to the discovery of a novel inhibitor molecule (QYT‐4h) against LdMetAP1. The compound QYT‐4h showed nearly 20‐fold less potency for human MetAP1 and had drug‐like features. Time–course kinetic assays suggested QYT‐4h acting through a competitive mode by binding to the metal‐activated catalytic site. Notably, QYT‐4h was most potent against the physiologically relevant Mn(II) and Fe(II) supplemented forms of LdMetAP1 and less potent against Co(II) supplemented form. Surface plasmon resonance and fluorescence spectroscopy demonstrated high affinity of QYT‐4h for LdMetAP1. Through molecular modelling and docking studies, we found QYT‐4h binding at the LdMetAP1 catalytic pocket occupying both the catalytic and substrate binding sites mostly with hydrogen bonding and hydrophobic interactions which provide structural basis for its promising potency. These results demonstrate the feasibility of employing small‐molecule inhibitors for selective targeting of LdMetAP1 which may find use to effectively eliminate leishmaniasis.
中文翻译:

开发基于喹啉的杂合体作为多诺瓦利什曼原虫甲硫氨酸氨肽酶 1 的抑制剂
甲硫氨酸氨肽酶 1 (MetAP1) 是针对许多对手的药物发现靶点,并且因其在 N 端甲硫氨酸加工中的作用而成为潜在的抗利什曼病靶点。为了从杜氏利什曼原虫( Ld MetAP1) 中发现针对甲硫氨酸氨肽酶 1 的新抑制剂,我们合成了一系列基于喹啉的杂合体,即 (Z)-5-((Z)-苄烷)-2-(喹啉‐3-ylimino)thiazolidin-4-ones (QYT-4a-i) 其体外筛选导致发现了一种针对Ld的新型抑制剂分子 (QYT-4h)元AP1。化合物 QYT-4h 对人类 MetAP1 的效力降低了近 20 倍,并且具有类似药物的特征。时程动力学分析表明 QYT-4h 通过与金属活化的催化位点结合以竞争模式起作用。值得注意的是,QYT-4h 对Ld MetAP1的生理相关 Mn(II) 和 Fe(II) 补充形式最有效,而对 Co(II) 补充形式的效力较低。表面等离子体共振和荧光光谱表明 QYT-4h 对Ld MetAP1具有高亲和力。通过分子建模和对接研究,我们发现 QYT-4h 结合在LdMetAP1 催化口袋占据催化和底物结合位点,主要通过氢键和疏水相互作用为其有前途的效力提供结构基础。这些结果证明了使用小分子抑制剂选择性靶向Ld MetAP1的可行性,这可能会用于有效消除利什曼病。
更新日期:2020-08-20
中文翻译:

开发基于喹啉的杂合体作为多诺瓦利什曼原虫甲硫氨酸氨肽酶 1 的抑制剂
甲硫氨酸氨肽酶 1 (MetAP1) 是针对许多对手的药物发现靶点,并且因其在 N 端甲硫氨酸加工中的作用而成为潜在的抗利什曼病靶点。为了从杜氏利什曼原虫( Ld MetAP1) 中发现针对甲硫氨酸氨肽酶 1 的新抑制剂,我们合成了一系列基于喹啉的杂合体,即 (Z)-5-((Z)-苄烷)-2-(喹啉‐3-ylimino)thiazolidin-4-ones (QYT-4a-i) 其体外筛选导致发现了一种针对Ld的新型抑制剂分子 (QYT-4h)元AP1。化合物 QYT-4h 对人类 MetAP1 的效力降低了近 20 倍,并且具有类似药物的特征。时程动力学分析表明 QYT-4h 通过与金属活化的催化位点结合以竞争模式起作用。值得注意的是,QYT-4h 对Ld MetAP1的生理相关 Mn(II) 和 Fe(II) 补充形式最有效,而对 Co(II) 补充形式的效力较低。表面等离子体共振和荧光光谱表明 QYT-4h 对Ld MetAP1具有高亲和力。通过分子建模和对接研究,我们发现 QYT-4h 结合在LdMetAP1 催化口袋占据催化和底物结合位点,主要通过氢键和疏水相互作用为其有前途的效力提供结构基础。这些结果证明了使用小分子抑制剂选择性靶向Ld MetAP1的可行性,这可能会用于有效消除利什曼病。


























 京公网安备 11010802027423号
京公网安备 11010802027423号