Tetrahedron ( IF 2.1 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.tet.2020.131523 Kseniya V Belyaeva 1 , Lina P Nikitina 1 , Andrei V Afonin 1 , Boris A Trofimov 1
|
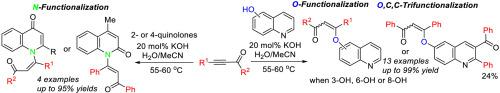
The expected one-pot multiple functionalization of hydroxyquinolines and quinolones with acylacetylenes (20 mol% KOH, 5 equiv. H2O, MeCN, 55–60 °C), which, according to the previous finding, might involve the addition of OH and NH-functions to the triple bond and insertion of acylacetylenes into the quinoline scaffold, retains mainly on the formation of chalcone-quinoline ensembles in up 99% yield. The higher functionalized quinolines can be obtained in a synthetically acceptable yield, when the above ensembles are treated with the second molecule of acylacetylenes. Thus, the further insertion of second molecule of the acetylenes into the quinoline scaffold occurs as a much slower process indicating a strong adverse substituent effect of the remote chalcone moiety.
中文翻译:

羟基喹啉和喹诺酮的多重官能化中的酰基乙炔。
羟基喹啉和喹诺酮与酰基乙炔(20 mol% KOH, 5 equiv. H 2 O, MeCN, 55–60 °C)的预期一锅多官能化,根据先前的发现,这可能涉及添加 OH 和NH-对三键和将酰基乙炔插入喹啉支架的作用,主要保留在查尔酮-喹啉集合体的形成中,产率高达 99%。当用第二个酰基乙炔分子处理上述集合体时,可以以合成可接受的产率获得更高官能化的喹啉。因此,乙炔的第二分子进一步插入喹啉支架中的过程要慢得多,这表明远程查尔酮部分具有强烈的不利取代基效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号