Molecular Cell ( IF 16.0 ) Pub Date : 2020-08-20 , DOI: 10.1016/j.molcel.2020.08.002 Dan Huang 1 , Cristel V Camacho 2 , Rohit Setlem 2 , Keun Woo Ryu 2 , Balaji Parameswaran 2 , Rana K Gupta 3 , W Lee Kraus 2
|
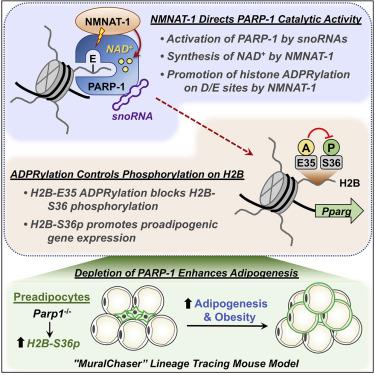
Although ADP-ribosylation of histones by PARP-1 has been linked to genotoxic stress responses, its role in physiological processes and gene expression has remained elusive. We found that NAD+-dependent ADP-ribosylation of histone H2B-Glu35 by small nucleolar RNA (snoRNA)-activated PARP-1 inhibits AMP kinase-mediated phosphorylation of adjacent H2B-Ser36, which is required for the proadipogenic gene expression program. The activity of PARP-1 on H2B requires NMNAT-1, a nuclear NAD+ synthase, which directs PARP-1 catalytic activity to Glu and Asp residues. ADP-ribosylation of Glu35 and the subsequent reduction of H2B-Ser36 phosphorylation inhibits the differentiation of adipocyte precursors in cultured cells. Parp1 knockout in preadipocytes in a mouse lineage-tracing genetic model increases adipogenesis, leading to obesity. Collectively, our results demonstrate a functional interplay between H2B-Glu35 ADP-ribosylation and H2B-Ser36 phosphorylation that controls adipogenesis.
中文翻译:

组蛋白 H2B ADP-核糖基化和磷酸化之间的功能相互作用控制脂肪生成。
尽管 PARP-1 对组蛋白的 ADP 核糖基化与基因毒性应激反应有关,但其在生理过程和基因表达中的作用仍然难以捉摸。我们发现小核仁 RNA (snoRNA) 激活的 PARP-1 对组蛋白 H2B-Glu35 的NAD +依赖性 ADP 核糖基化抑制了 AMP 激酶介导的相邻 H2B-Ser36 的磷酸化,这是促脂肪生成基因表达程序所必需的。PARP-1 对 H2B 的活性需要 NMNAT-1,一种核 NAD +合酶,可将 PARP-1 催化活性引导至 Glu 和 Asp 残基。Glu35 的 ADP 核糖基化和随后 H2B-Ser36 磷酸化的减少抑制了培养细胞中脂肪细胞前体的分化。Parp1在小鼠谱系追踪遗传模型中敲除前脂肪细胞会增加脂肪生成,导致肥胖。总的来说,我们的结果证明了 H2B-Glu35 ADP-核糖基化和控制脂肪生成的 H2B-Ser36 磷酸化之间的功能相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号